Cell technology topic: rat optic nerve oligodendrocyte culture experiment
2022-11-10 14:06:23
Rat optic nerve oligodendrocyte culture can: (1) obtain rat optic nerve oligodendrocytes; (2) for optic nerve damage repair research; (3) for oligodendrocyte related research.
experimental method
- Bovine serum culture
| Principle of experimental method | The optic nerve, DMEM/F12 conditioned medium was cultured, and the morphology and growth of the cells were observed, and immunohistochemical identification was performed. |
|---|---|
| Experimental Materials | Wistar rat |
| Reagents, kits | Sodium selenite putrescine transferrin insulin thyroxine progesterone glutamine calf serum rabbit anti-mouse GC antibody |
| Instruments, consumables | Ophthalmic scissors dropper centrifuge Petri dish CO2 incubator |
| Experimental procedure | First, the experimental steps 1. Ten newborn Wistar rats were taken for 2 days, and the bilateral optic nerves were removed under sterile conditions. 2. Inoculate onto a poly-lysine-treated coverslip in a 24-well culture plate. 3. DMEM/F12 medium containing 30 g·L -1 calf serum was added, and cultured at 37 ° C, 50 mL/L CO 2 , 100% humidity for 3 d. 4. After 3 d, discard the waste liquid and continue to culture with 5 g·L -1 calf serum DMEM/F12 conditioned medium. 5. Change the fluid twice a week. After obtaining a sufficient number of cells, the culture was continued with a serum-free conditioned medium, and the solution was changed every 2 days. Second, morphological observation The microscope was inverted every day to observe the growth process and morphological changes of the cultured cells and photographed. Third, immune cell chemistry identification 1. Remove the cell-containing coverslip and rinse it 3 times × 5 min in 0.01 mol·L -1 PBS. 2. Fix in cold acetone for 10 min. 3. Rinse with PBS (3 times x 5 min). 4. Incubate 30 g·L -1 hydrogen peroxide for 15 min at room temperature. 5. Rinse PBS 3 times x 5 min. 6. Add normal goat serum and incubate for 20 min at room temperature. 7. Add 1:100 GC antibody, negative control with PBS instead of primary antibody, incubate for 60 min at 37 °C. 8. Rinse PBS 3 times x 5 min. 9. Biotinylated goat anti-rabbit IgG was added dropwise at 37 ° C for 20 min. 10. Rinse PBS 3 times x 5 min. 11. Add horseradish enzyme-labeled chain enzyme avidin working solution. 12. DAB working solution develops color, and tap water stops the reaction. 13. Dehydrated, transparent, DPX mounts are preserved. Fourth, the results Morphological observation The tissue block began to adhere at 24 h, and a small amount of cells were seen at the edge of the nerve tissue at 48-72 h, mainly in the shape of a circle or a fusiform (Fig. 1). Around 8 to 9 days, the cells are basically covered with culture holes. At this time, it was purified by using serum-free conditioned medium culture. Some cells died during the culture. The cell body obtained around 12 d is basically round or polygonal, and the diameter is about 6-10 μm. The nucleus is larger and the cytoplasm is less (Fig. 2). 2. Immunohistochemical staining results Immunohistochemical staining of cultured optic nerve oligodendrocytes was positive for GC protein, and negative for primary antibody. The staining results showed that the mature oligodendrocyte cells were rich in protrusions and formed a spider web shape (Fig. 3). Hematoxylin counterstained, less heterogeneous cells, more than 90% of positive cells (Figure 4). 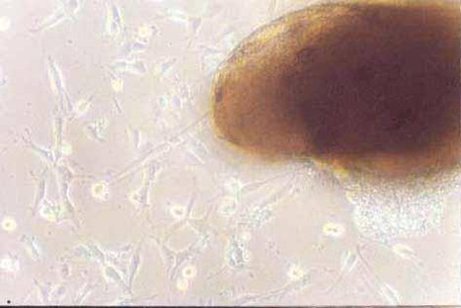 Figure 1 Cells migrated from optic nerve tissue at the third day (10×10) Figure 1. On day 3 of culture, cells migrated from the optic nerve (10×10) 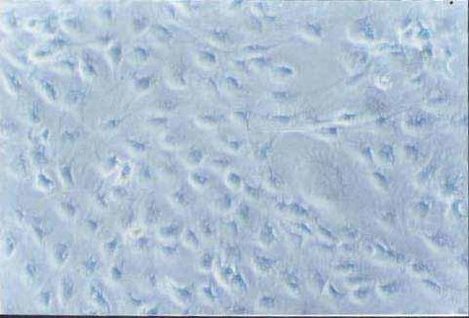 Figure 2 Purified oligodendrocytes of optic nerve at the fifteenth day (10×40) Figure 2. Purified optic nerve oligodendrocytes (10×40) on day 15 of culture 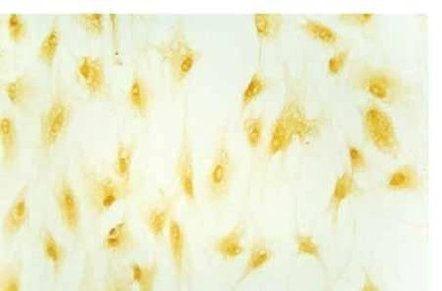 Figure 3 Positive expression of GC in oligodendrocytes at the seventh day (10×20) Figure 3. GC-positive expression of oligodendrocytes on day 7 of culture (10×20) 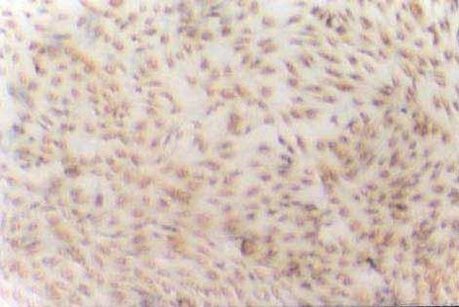 Figure 4 Positive expression of GC in purified oligodendrocytes at the fifteenth day (hematoxylin staining) (10×20) Figure 4. GC-positive expression of purified oligodendrocytes (hematoxylin counterstain) on day 15 of culture (10×20) |
| other | I. Discussion The discovery of the inhibition of the nerve regeneration gene Nogo brings hope for the repair of visual nerve damage and the study of visual function protection. Optic glial cells include two types of astrocytes and oligodendrocytes, which have different differentiation and different functions. Mature oligodendrocytes no longer have the ability to divide and proliferate, which is the terminal cell of division [1] , and it is difficult to culture. In 1980, McCarthy [2] established an isolation culture model for isolating and purifying astrocytes and oligodendrocytes from brain gray matter tissues. At present, the law is widely used at home and abroad [3] . Separation and culture were carried out by vibration separation method using the difference in the ability of oligodendrocytes and astrocytes to adhere. This method is mainly used to obtain astrocytes, and fewer oligodendrocytes are obtained. There are many disadvantages such as the fact that the operation steps are easily contaminated, and the cells are killed due to mechanical damage caused by the oscillation and centrifugation during the separation process. In this experiment, optic nerve tissue lumps were used to condition the primary culture of neonatal rat optic glial cells. Different conditions were used to purify oligodendrocytes and astrocytes. cell. Oligodendrocytes and type II astrocytes are derived from an ordinary bipotent oligodendrocyte-2 astrocyte progenitor cell [1] . About 1 week after birth, oligodendrocytes mature gradually. Therefore, O2A progenitor cells can be obtained from newborn rats. In vitro studies have found that thyroxine, insulin, transferrin, putrescine, progesterone and trace element selenium can cause O2A progenitor cells to differentiate into oligodendrocytes. Serum and the like can induce the differentiation of O2A progenitor cells into type II astrocytes [1, 4] . We use this feature to gradually reduce the serum concentration in conditioned medium and promote the differentiation of O2A progenitor cells into oligodendrocytes. Finally, serum-free conditioned medium was used to inhibit the proliferation of astrocytes and other heterogeneous cells, while oligodendrocytes were not affected under these conditions. GC is a major lipid expressed in the oligodendrocyte membrane and myelin, which is a specific chemical marker for the common application of oligodendrocytes [1, 3] . The results of immunostaining identification showed that the purity was higher than 90%. This method is simple, reliable, and easy to generalize, and is convenient for research on oligodendrocyte related topics. Second, the literature 1. He Ping, Shen Xinya. Progress in the proliferation and differentiation of oligodendrocytes [J], Journal of Neuroanatomy, 2000, 16(2): 183-188. 2. McCarthy KD, DeVellis J. Preparation of separate astroglial and oligodendroglia cell cultures from rat cerebral tissue [J], J Cell Biol 1980, 85: 890-902. 3. Wu Yamin, Ma Haihan, Liao Weihong. Purification, culture and identification of rat astrocytes and oligodendrocytes [J], Trauma Surgery 2000, 4: 207-209. 4. Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium [J], Nature 1983, 303 (5916): 390-6. |
Sunflower Seeds,Sunflower Kernels,Bulk Sunflower Seeds,Shelled Sunflower Seeds
Wuyuan county dafeng oil food co.,ltd , https://www.dafengfood.com.cn