Antibiotic detection solution in chicken
2022-08-15 08:12:44
Recently, the media exposed KFC's big supplier - Shanxi Suhai Group "45 days of eating chicken" incident. A chicken is fed with feed and medicine, and it takes only 45 days from hatching to serving on the table! According to the report, as many as 11 antibiotics were fed to broilers during the 11 days of the culture phase. This news has caused widespread concern and discussion on the web.
Antibiotics are a class of metabolites produced by microorganisms (including bacteria, fungi, actinomycetes) or higher animals and plants that are resistant to pathogens or other activities during their life. Antibiotics can interfere with the developmental functions of other living cells. In the 1940s, scientists discovered that the addition of antibiotics or their fermentation residues to feed can promote the growth of livestock. In 1950, the US Food and Drug Administration (FDA) first approved the addition of antibiotics to feed. Later, countries around the world conducted antibiotic feeding tests and used them for production. The Ministry of Agriculture of the People's Republic of China has promulgated the “Provisions for the Use of Veterinary Drugs and Means for Feeding Pharmaceutical Additivesâ€. The types of antibiotics allowed for use as feed additives are available in three categories and 30 types, and are strictly applied to applicable animals, minimum dosage, maximum dosage and withdrawal period. Provisions. It can be said that the normal addition of antibiotics is completely legal and reasonable. It is illegal to add antibiotics that are not in compliance with the Regulations on the Use of Veterinary Drugs and Uses for Feed Pharmaceutical Additives.
At present, the common illegal addition of antibiotics is mainly the excessive use of antibiotics. The common antibacterial antibiotic, chlortetracycline, is used as an example. The provisions on the variety and use of veterinary drugs allowed for feed pharmaceutical additives are pointed out. The use of food animals (including broilers) The upper limit is 10 weeks, the maximum dosage is 50 million units/ton (compound feed), and the supplement requires that the low-calcium feed should not be used for more than 5 days at high dosages. Excessive use can lead to excess chlortetracycline in chicken. The Ministry of Agriculture's Maximum Residue Limits for Veterinary Drugs in Animal Foods (Announcement No. 235 of the Ministry of Agriculture, 2002) requires that the residual amount of chlortetracycline in chicken meat should not exceed 100 μg/kg. The General Administration of Quality Supervision, Inspection and Quarantine also issued the standard "GB/T 20764-2006 Determination of oxytetracycline, tetracycline, chlortetracycline, doxycycline residues in the muscles of edible animals, liquid chromatography-ultraviolet detection method", clear detection Methods and limits of detection.
According to "GB/T 20764-2006", RephiLe specially introduced a solution for chlortetracycline detection in chicken. The detection limit was 0.005 mg/kg by HPLC. The detailed solution is as follows:
1. Scope of application
It is suitable for the determination of chlortetracycline residues in beef, lamb, pork, chicken and rabbit meat.
2. Principle
The chlortetracycline residue in the muscle of the animal was extracted with 0.1 mol/L Na 2 EDTA-Mcllvaine (pH=4.0±0.05) buffer. After the extract was centrifuged, the supernatant was exchanged with Oasis HLB solid phase extraction column and carboxylic acid type cation. Column purification, HPLC detection, external standard method for quantification.
3. Sample extraction
Take 1 kg of the sample, fully mash it, mix it, divide it into two parts, and place it in a clean container and seal it into a sample. Store frozen at -18 °C. Weigh 6.00 g ± 0.01 g of the sample into a 50 mL stoppered centrifuge tube and add 30 mL of Na 2 EDTA-Mcllvaine buffer solution. After homogenization for 1 min, the ice bath was sonicated for 10 min. The extract was centrifuged at 10,000 r/min for 10 min, and the supernatant was poured into another centrifuge tube. The residue was added to the 20 mL buffer solution and extracted once. Combine the supernatant.
4. Purification
The sample extraction supernatant was added to an Oasis HLB solid phase extraction column at a flow rate of ≤ 3 mL/min. After the supernatant was completely discharged, the column was washed with 5 mL of methanol + water (1 + 19) and dried by a vacuum pump for 40 minutes. Discard the effluent. Elution with 15 mL of ethyl acetate and the eluent was collected in a 100 mL flask.
The above eluate was passed through a carboxylic acid type cation exchange column at a flow rate of ≤ 3 mL/min under reduced pressure. After the eluate completely flowed out, the column was washed with 5 mL of methanol and dried with a vacuum pump for 5 minutes. Discard the effluent. Elution was carried out with 4 mL of mobile phase: acetonitrile + methanol + 0.01 mol/L oxalic acid solution (2+1 + 7), and the eluate was collected in a 5 mL sample tube. Make up to 4mL. Filter with a 13 mm, 0.22 μm PTFE needle filter.
5. Chromatography
Column: Mightsil RP-18 GP 3μm (150mm × 4.6 mm);
Mobile phase: acetonitrile + methanol + 0.01 mol / L oxalic acid solution (2 + 1 + 7)
Flow rate: 0.5 mL/min;
Column temperature: 25 ° C;
Injection volume: 60 μL.
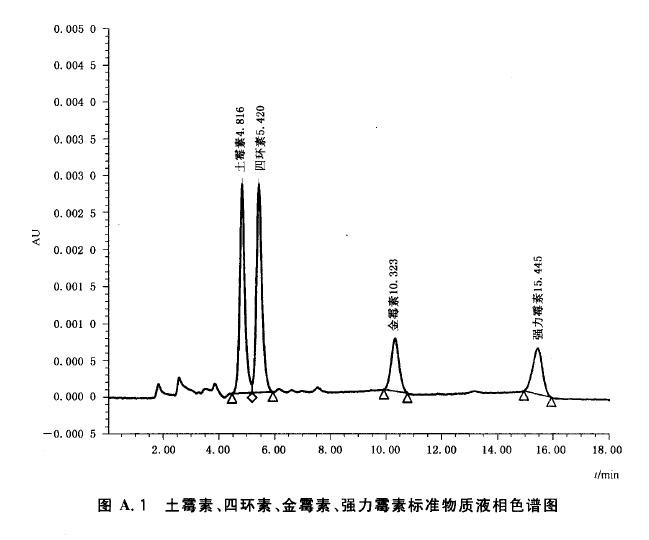
In order to ensure the accuracy of the experiment, the liquid phase water must use the first grade water in accordance with the People's Republic of China standard GB 6682-2008. The configuration of the other various standard solutions requires a minimum of secondary water. RephiLe's Direct-Pure EDI and PURIST Classic Kits provide both high purity water and ultrapure water for a full range of laboratory water needs. RephiLe Direct-Pure EDI high-purity water system uses Siemens' EDI module to provide stable and high-quality high-purity water with tap water as the inlet water, and the products have reached the advanced level of similar products. PURIST series ultra-pure water system, exquisite and compact, easy to use, pure water as water, production of ultra-pure water, water quality exceeds or meets GB and ASTM, CAP, CLSI and other first-class water standards. RephiLe pure water system has passed FCC, CE and other certifications, as well as professional verification engineers, providing the required verification service support, and the 3Q certification required by various standards, helping users to pass GMP, GLP, etc. System certification can ensure users have no worries about water use. In order to thank the majority of users for their love, RephiLe launched a special promotion at the end of 2012. Each set of classic sets is only for 40888 yuan, and there are more purchases. See http:// for details. In addition, RephiLe also provides Direct Pure UP, an integrated pure water system that uses tap water as the influent water. It can simultaneously produce RO and ultrapure water for customers to choose.
In order to better protect the HPLC instrument, the solution should be pre-filtered before loading to remove particles and microorganisms in the solution to prevent blockage of the column and damage to the pump, thus ensuring the operation performance of the instrument and prolonging the service life of the instrument. RephiLe offers a variety of needle filters, such as PES, PTFE, Nylon, MCE, for chromatographic sample filtration.
RephiLe related product information:
Item number | name | description |
RS2200QUV | PURIST UV | RephiLe ultra pure water system |
RD0E01000 | Direct-Pure EDI 10 | RephiLe high purity water system |
RJF1345NH | RephiLe needle filter | 13 mm PTFE membrane, 0.45 μm pore size, 100 pcs/package |
RJF3245NH | RephiLe needle filter | 32 mm PTFE membrane, 0.45 μm pore size, 100 pcs/package |
RASYR2050 | Male Luer-Lok Lock Syringe | 20 ml, 50 sticks / package |
RASYR5020 | Male Luer-Lok Lock Syringe | 50 ml, 20 sticks / package |
Blood pressure raw healthy green material
Blood pressure raw healthy green material,Velpatasvir Price, Iodixanol Solubility,Iopromide Api Manufacture,Hydroxycitric Acid for Skin.
Human APIs - Blood Pressure raw , APIs Raw powder , Antipyretic And Analgesic , Antibiotic & Antibacterial
Now we have 3 GMP standard workshop, Meanwhile, the factory is equipped with the researching and quality inspection centre, with strong technology research and development strength. We also have 3 salesdepartments over 30 people and sell our products all over the world.
For customer`s needs, OEM service is also acceptable. If you have a good idea in new product production but lack of laboratory device and human resource, we are glad to solve this problem for you. Sincerely hope to strengthen exchanges and cooperation with friends from both home and abroad.
Blood pressure raw healthy green material,Velpatasvir Price, Iodixanol Solubility,Iopromide Api Manufacture,Hydroxycitric Acid for Skin
Xi'an Hollysince Biotech Co., Ltd. , https://www.hollysince.com