[Dry goods] Live imaging - let the tumor cells have nowhere to shape
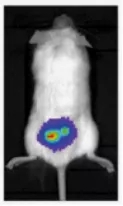
Figure 1. In vivo imaging [1]
As early as 1999, Dr. Weissleder (Figure 2) of Harvard University of the United States pioneered the concept of molecular imaging (MI), which applied imaging methods to qualitatively and at the cellular and molecular levels of biological processes in vivo. Quantitative Study. In vivo imaging is based on molecular imaging. Through this imaging system, it is possible to observe the growth and metastasis of tumors in living animals, the development of infectious diseases, and the expression of specific genes.

Figure 2. Ralph Weissleder [2]
In recent years, China's medical and health industry has developed by leaps and bounds, and new drug research and development has gradually become a key research area for research institutions and pharmaceutical companies. Live imaging has contributed a valuable force to drug research and development. At present, the experimental methods applied to the development of small animal living body imaging technology mainly include bioluminescence and fluorescence .
Bio-luminescence based in vivo imaging technology method and application
Bioluminescence is mainly the labeling of genes, cells and living animals using Luciferase. The method of labeling cells is basically to insert a gene of luciferase into the chromosome of a cell to be observed by molecular biological cloning technology, and to screen a cloned cell to culture a cell line stably expressing luciferase. The cell line is then transferred to a specific mouse to form a model. Injection of fluorescein (a substrate for luciferase) into model mice maintains luciferase-labeled cell luminescence in vivo for 30-45 minutes. Because each luciferase-catalyzed reaction produces only one photon, which is unobservable by the naked eye, an advanced imaging system is required, followed by a highly sensitive cooled CCD camera and a specially designed imaging black box and imaging software. Observe and record these photons (Figure 3). [1,3,4]
Briefly, luciferase reacts with a substrate to produce chemiluminescence. This light comes from a chemical reaction and does not require excitation light. The instrument records the number of photons detected per unit time, which is equivalent to the direct quantitative recording of the number of cells expressing luciferase.
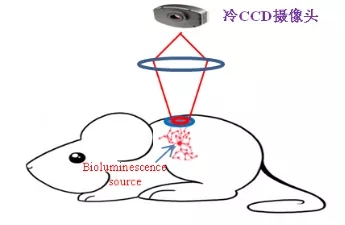
Figure 3. Capturing a light source with a cooled CCD camera
Figure 4. In vivo imaging of intravenous injection of Luciferase-labeled multiple myeloma in mice [5]
Fluorescence-based in vivo imaging technology method and application
Unlike bioluminescence, fluorescence technology is labeled with fluorescent reporter genes (GFP, RFP, etc.) or fluorescent dyes (including novel nanolabel materials such as fluorescent quantum dots), which can be formed by fluorescence generated by reporter genes, fluorescent proteins or dyes. Biological light source in the body. Bioluminescence is the autofluorescence of an animal. It does not require excitation of the light source, and fluorescence requires excitation from an external excitation source before it can be detected by the imaging system. Fluorescent labels are widely used, such as animals, cells, microorganisms, antibodies, drugs, nanomaterials, etc. [1,3,4] .
Next, I will take a look at a set of experiments using fluorescence technology: To study the tumor targeting of model proteins, a near-infrared fluorescent molecule, indocyanine green (ICG), is loaded with PDPA (human serum albumin surface-fixed). Nano-fluorescent probe (HDI) is formed by in situ growth of a polymer chain with pH responsiveness. The ICG is entrapped in the micelle nucleus and undergoes fluorescence aggregation quenching, but when it reaches the tumor, it is weakly acidic due to the tumor microenvironment. The micelles disintegrate, the ICG is released to restore fluorescence, and the protonation of PDPA is positively charged, which facilitates its adsorption on the cell surface and endocytosis, thereby achieving tumor-targeted fluorescence imaging. This study also showed that the introduction of pH-responsive polymer chains on the surface of proteins can improve protein targeting. Pharmaceutical proteins can use this technology to improve drug efficacy and reduce side effects, while the responsive protein-polymer conjugates The assembly system can also be used as a small molecule drug carrier, thus expanding the clinical application of protein drugs and small molecule drugs [6] . (Figure 5)
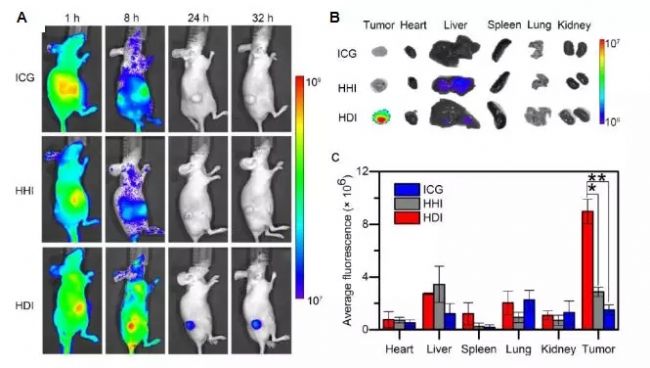
Figure 5. Fluorescence imaging of C8161 melanoma in vivo and in vitro after intravenous injection of HDI nanofluorescent probe in mice [6]
Although the fluorescence signal is much stronger than bioluminescence, the background noise generated by non-specific fluorescence makes the signal-to-noise ratio much lower than that of bioluminescence. The specific advantages and disadvantages of the two are shown in Table (1). For different studies, the appropriate method can be selected according to the characteristics of the two and the experimental needs [7] . Â
Table I)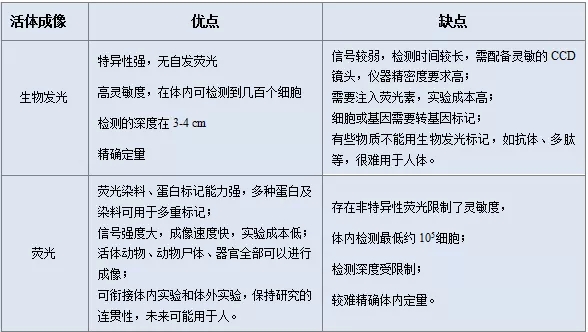
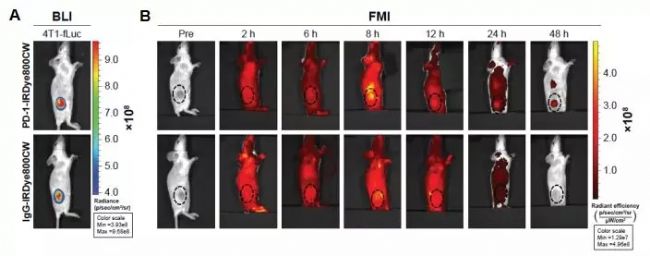
Figure 6. FMI detection of PD-1-IRDye800CW biodistribution in mice bearing 4T1-Luc breast tumors [8]
The key point is that Bio-Syracuse currently has a self-developed B-IL17-EGFP knock-in mouse. All mouse cells expressing IL17 express green fluorescent protein (EGFP) at the same time to study the cytokine IL-17. A series of chronic diseases [9] . The pharmacological potency platform has a variety of GFP-Luciferase cell lines and tumor models , which can be used to establish subcutaneous and orthotopic tumor models or to establish metastatic tumor models, and to carry out in vivo pharmacological efficacy services for the corresponding models [10] . The gene editing platform can provide a variety of reporter genes (such as EGFP, mRFP, mCherry, mYFP or LacZ) to the mouse (the location of the target gene is introduced into the reporter gene), which facilitates the study of the transcriptional activity of the target gene promoter and monitors the target protein. Expression localization and cell function [11] . If you need, welcome your friends to consult in detail!
[4] Li Dongmei, Wan Chunli, Li Inheritance. Research progress on small animal living imaging technology. Chinese Journal of Biomedical Engineering. 2009
[5] Delmore JE, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011 Sep 16;146(6):904-17.
[6] LiP, SunM, XuZ, LiuX, ZhaoW, GaoW. Site-Selective in Situ Growth-Induced Self-Assembly of Protein-Polymer Conjugates into pH-Responsive Micelles for Tumor Microenvironment Triggered Fluorescence Imaging. Biomacromolecules. 2018 Nov 12;19( 11): 4472-4479.
[7]https://baike.baidu.com/item/ visible light imaging technology
[8] Yang Du, et al. Improved resection and prolonged overall survival with PD-1-IRDye800CW fluorescence probe-guided surgery and PD-1 adjuvant immunotherapy in 4T1 mouse model.Int J Nanomedicine. 2017; 12: 8337–8351.
[9]http://
[10]http://
[11]http://

Scan to pay attention to the 100 Olympics map for more information~
Single Use Trocar Site Closure Device
A fascial closure device is a laparoscopic instrument used to close the fascia, the layer of tissue that covers and separates abdominal organs and muscles. The device is used during laparoscopic surgery to create a secure closure of the fascia after the surgical procedure is complete.
The fascial closure device consists of a needle and suture material attached to a handle. A needle is used to puncture the fascia, then suture material is passed through the puncture site and tied tightly to close the fascia. The device's handle is used to control the movement of the needle and suture material, allowing precise placement and closure of the fascia.
The use of fascial closure devices during laparoscopic surgery can help reduce the risk of postoperative complications such as hernias, wound infections, and intestinal obstruction. It also closes the fascia more quickly and efficiently, reducing the overall time required for the procedure.
Single Use Trocar Site Closure Device,Medical Use Fascial Closure System,Single Use Fascial Closure System,Exit Fascia Closure Device
Changzhou Weipu Medical Devices Co., Ltd. , https://www.cnweipumedical.com