Microwave digestion solution for metal impurity detection
The presence of impurities in pharmaceuticals is of deep concern not only because of the toxicity of certain contaminants, but also because they may adversely affect the stability and shelf life of the drug, or may cause harmful side effects. Therefore, it is necessary to use the raw materials, intermediates and active ingredients (API), excipients (stabilizers, fillers, binders, colorants, flavoring agents, sugar coatings, etc.) used in the production of pharmaceuticals, as well as in the final pharmaceutical products. Organic and inorganic (element) impurities are monitored and controlled. Impurities that may be introduced during the production process, such as catalysts and contaminants from production process equipment, must also be monitored.
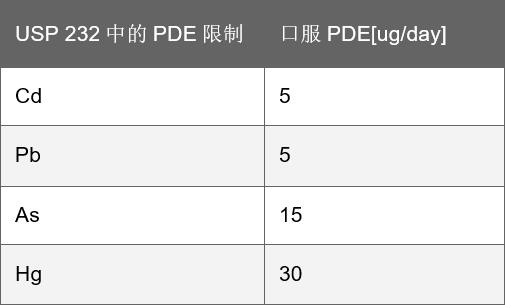
Beginning in January 2018, the ICH Guidebook Q3D chapter4, USP232,233 was officially implemented. The guidelines are divided into four categories based on the toxicity of elemental impurities and their potential for toxicity in the drug – 1, 2A, 2B and 3, and specify the type of element, dosage form (oral, injection and inhalation). As well as allowing daily contact volume (PDE).
Of note, cadmium (Cd), lead (Pb), arsenic (As), mercury (Hg) and cobalt (Co) in grade 2, vanadium (V), niobium (Ni) in grade 1. They are human poisons and all PDEs are low. For these elements, even if there is no artificial addition, a risk analysis must be performed to prevent exceeding their PDE. Based on these assessments, a reasonable control strategy is defined, from no analysis to regular research, to rational testing of the final product.
Difficulties in pre-treatment of heavy metals in pharmaceuticals
With the development of the economy, generic drugs are no longer the only way out for the domestic pharmaceutical industry, and more and more pharmaceutical companies are constantly exploring the development of new drugs. The active pharmaceutical ingredients (API) often have difficulties in heavy metal analysis. Due to the stable and complex structure of these drugs, the general pretreatment method is often difficult to meet the requirements, and there will be a phenomenon of flocculation after constant volume, which is caused by the complete digestion of the sample. For the pretreatment of such samples, pretreatment with ashing is more common. However, the detection of some volatile elements such as arsenic (As) and mercury (Hg) cannot be performed. To this end, Anton Paar has released a new product, the Anton Paar Multiwave 7000 Super Microwave Digestion System, to provide a pre-treatment solution for these drugs.
Anton Paar's Multiwave 7000 Super Microwave Digestion System provides pharmaceutical industry users with the sample preparation solutions they need to capture accurate trace analysis results, handling demanding and complex samples. It is the product of an excellent design part of HPA combined with modern microwave digestion technology. Pressure-tight reaction tubes and vessels are used in the pressurized digestion chamber (PDC). It has: pre-pressurized design; unparalleled working conditions; is not limited by the temperature or pressure limits of the system; does not have the advantages of traditional microwaves such as disposable glass digestion tubes. Let the "samples that cannot be digested" become a thing of the past. At the same time, Multiwave 7000 provides complete PQP files. The functional design also includes 21CFR Part11, such as audit tracking, user level management, electronic signature and other necessary modules for pharmaceutical companies. Easily respond to reviews by various agencies.

Application of Multiwave 7000 in pharmaceutical preparations
Experimental overview:
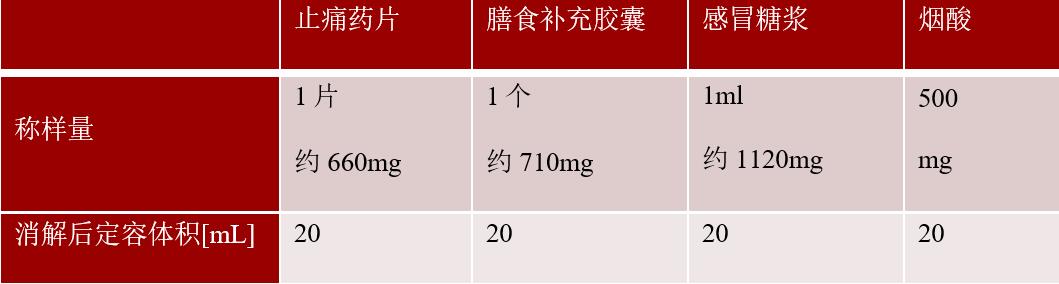
ï¶ Â· Samples: painkillers, dietary supplements, cold syrup, niacin nitrite · Elements to be tested: cadmium (Cd), lead (Pb), arsenic (As), mercury (Hg)
ï¶ Â· Sample weight:    Â
ï¶ Â·Digestion reagent: HNO3, HCl, HF
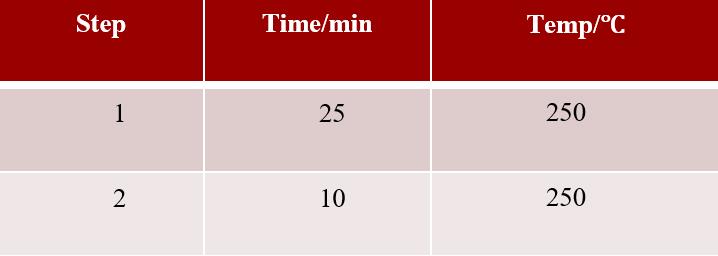
ï¶ Â· Pretreatment equipment: Anton Paar Multiwave 7000 Super Microwave System ï¶ Â· Digestion program:
ï¶ Â· Analytical equipment: ICP-MS, Agilent 7900
Experimental results:

Application of Multiwave 7000 in active pharmaceutical ingredients
Experimental overview:
ï¶ Â· Sample: ketoprofen, fenofibrate, amlixir, 4,6-dimethyl-ethyl-methylsulfonylpyridinium · sample weight: 0.1g
ï¶ Â·Digestion reagent: HNO3
ï¶ Â· Deconstruction procedure:
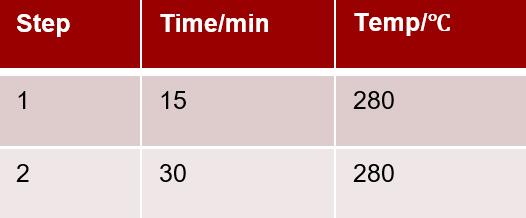
Experimental results:
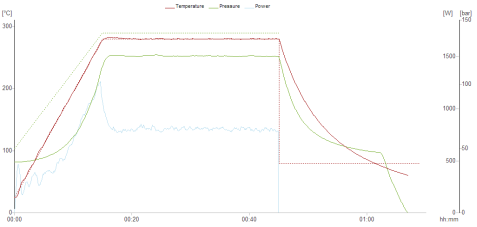
During the experiment, the temperature and pressure data are monitored in real time, which can accurately reflect the digestion state of the sample. The state after sample digestion is as follows:
| |
Before volume | After constant volume |
For more information on Multiwave Microwave Digestion/Extraction Solutions, please click on "Read the original", link to the website Microwave Digestion product page, live chat and online registration form
20K Ultrasonic Welding Machine
Product categories of 20K Ultrasonic Welding Machine, we are specialized manufacturers from China, Focus on Digital Ultrasonic Welding Machine solution for 16 years, high quality plastic ultrasonic welding machine wholesale high-quality machine of ultrasonic welding R & D and manufacturing, we have the perfect after-sales service and technical support. Look forward to your cooperation!
20K Ultrasonic Welding Machine ,Digital Ultrasonic welder,20K Plastic Ultrasonic Welding Machine
Wuxi DIZO Ultrasonic Technology Company , https://www.dizosonic.com

