Advantages and applications of His-tag, the preferred label for protein purification
His Tag Protein Purification Tool - Essential for Protein Purification
Polyhistidine-tagged recombinant proteins expressed in E. coli and other prokaryotic systems are typically purified by immobilized metal ion affinity chromatography or referred to as IMAC. In this process, the support (usually a beaded agarose gel) Or magnetic bead particles) Derivatized with a suitable coupling reagent, such as nitrilotriacetic acid (NTA) or iminodiacetic acid (IDA). These chelating groups will immobilize the desired divalent metal ion (for example) Nickel, copper, cobalt and zinc), then these metal ions are ultimately used as contact molecules and target molecules in the mixed protein. In choosing which ligand to use, you need to consider the binding capacity of the IMAC resin mainly depends on the nature of the protein being purified and the metal ions used in the purification process.

Highly purified His-tag fusion protein with NTA, IDA, and IMCA-type His-tag purified packing or prepacked column products
His-Tag affinity chromatography packing
His-Tag can interact with a variety of metal ions, such as Ca2+, Mg2+, Ni2+, Cu2+, Fe3+, etc. The principle is to use the surface properties of the protein to be adsorbed on the gel column to achieve separation and purification. The purpose of the protein.
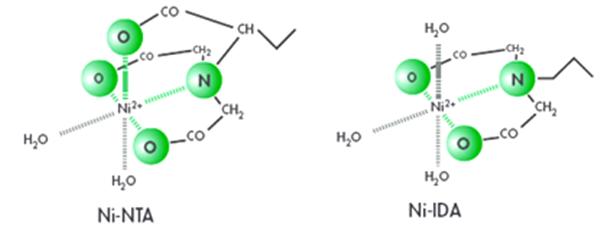
Ni2+ is most widely used in affinity purification experiments. Ni2+ affinity chromatography columns can be divided into two types according to the binding groups - Ni-IDA and Ni-NTA. Ni2+ has six chelating sites, of which Ni-IDA sequesters trivalent and Ni-NTA sequesters tetravalent. Therefore, the loading of IDA is higher than that of NTA. Under the same conditions, the concentration of imidazole in Ni-IDA elution is also higher than that of Ni-NTA. However, its weak binding force makes the metal ions easily leaching during the elution stage and tightly binds to the target protein, resulting in low protein yield, impure product and metal ion contamination. The NTA has a uniform particle size, a smaller particle size, and a more stable chelated nickel, which can withstand higher reducing agents, making the filler more stable and nickel ions not easily falling off.
His-tag is the preferred label for protein purification and its advantages are:
1. The N-terminal His-Tag is compatible with the transcriptional translation mechanism of bacteria, which is beneficial to protein expression;
2. It is easier to purify the His-Tag fusion protein by IMAC (immobilized metal ion affinity chromatography);
3. His-Tag has little effect on the properties of the target protein itself, and does not change the solubility and biological function of the target protein itself;
4. His-Tag is very small, and has no effect on the structure of the protein after crystallization of the fusion protein; the immunogenicity of His-Tag is relatively low, and the purified protein can be directly injected into the animal for immunization and preparation of the antibody;
5. Constructed as a parental tag with other affinity tags and can be applied to a variety of expression systems;
His-Tag fusion proteins are also available in a wide range of applications, either in the presence of nonionic surfactants or under denaturing conditions. The former is usually used to purify a hydrophobic protein of interest, while the latter is usually purified from inclusion bodies.
IMAC is relatively stable under normal conditions, but the presence of a chelating agent causes its metal ions to fall off. For example, some non-specific weak chelating agents are commonly found in the lysates of E. coli, such as the production of dicarboxylic acids in the tricarboxylic acid cycle. Thus, E. coli produces highly specific metal chelators (usually present in the periplasm of bacteria) under certain high pressure conditions, resulting in greatly reduced purity and yield of His-Tag fusion protein purification. Therefore, in the experiment of purifying the His-Tag fusion protein, it is first necessary to remove the chelating agent.
The PurKineTM His-Tag Purification System is based on an innovative high-load IMAC matrix that ensures efficient purification of His-Tag protein from total lysate in a single step. Our patented metal ion chelation scheme makes it compatible: reducing agents (such as DTT), chelation metalloproteinase inhibitors (such as EDTA), and most buffer substrates and salt environments. The PurKine product line is available in a variety of formats such as: free combination: chelating groups (IDA, NTA or Super NTA), cross-linking media (4% or 6% cross-linked agarose gel, magnetic beads), and metal ions ( Ni, Co, Cu, metal-free IMAC), a patented assembly chelating formulation that guarantees maximum yield, stability and solubility of His-tagged proteins.
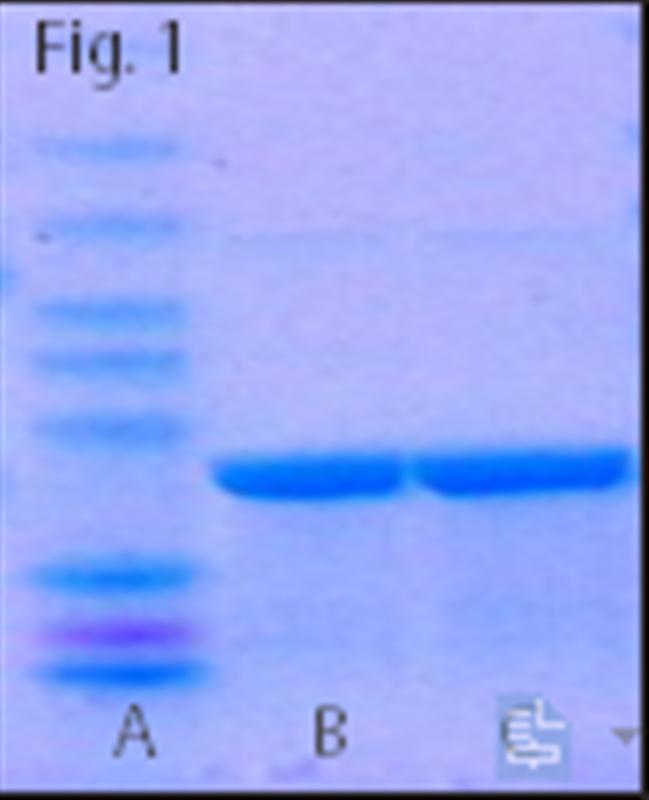
Fig. 1. His tag recombinant protein purification results. PurKineTM His-Tag Ni-NTA Resin 4FF (Lane B)
Corresponding Purified Products of Other Brands (Lane C)
PurKineTM His-Tag's purified fillers are also available in pre-packed columns and kits for added convenience. In addition, there are agarose gel resin fillers and magnetic beads.
PurKineTM His-Tag Ni-Super Resin is an excellent alternative to Ni-NTA Resin. Compared to NTA, Ni-Super and Ni have a tighter conformational conformation, more stable chelation, and a higher degree of compatibility with various chemicals. Such as reducing agents, chelating agents. PurKine? His-Tag Ni-Super Resin is especially suitable for the purification of His-Tag proteins in samples containing a medium that causes Ni ions to shed, such as a secreted protein sample solution containing a chelating agent. In addition, it has a high flow rate and is suitable for large-scale purification of proteins.
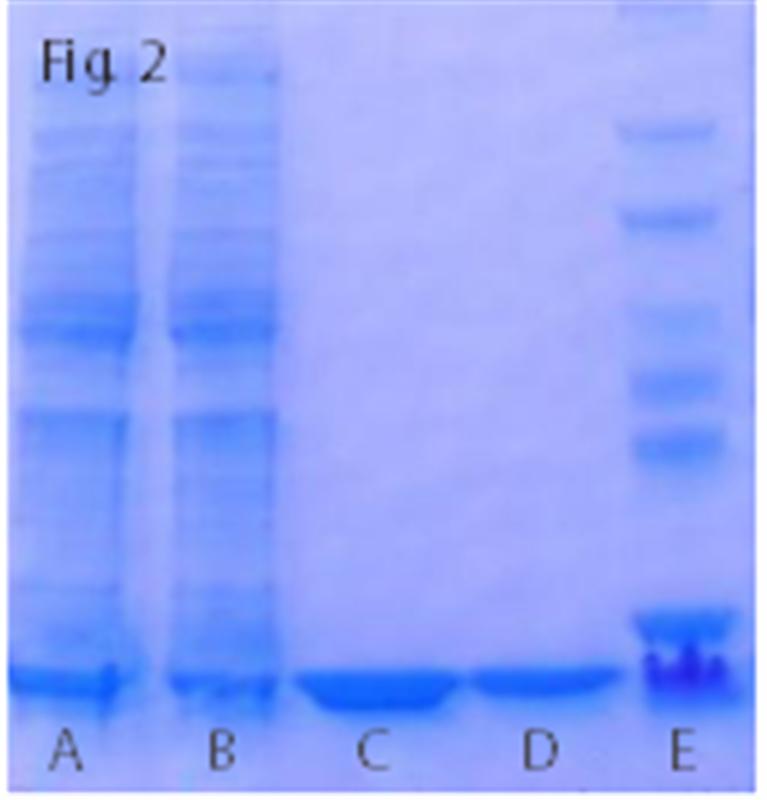
Fig.2. PurKine? His-Tag Ni-Super Resin is compatible with a wide range of chemicals: 0.01M HCl (Lane C), 0.01M NaCl (Lane D)
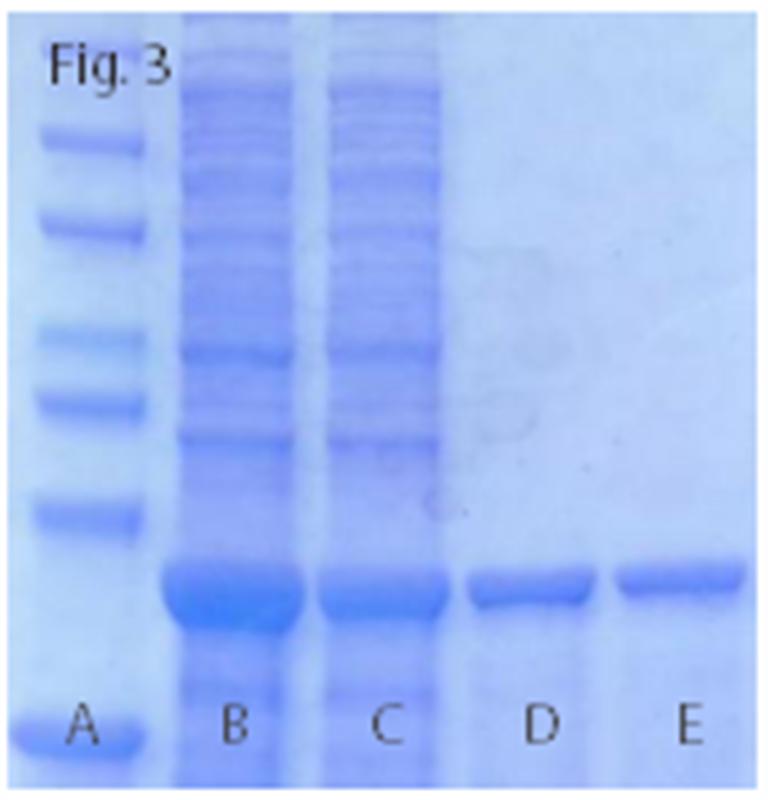
Fig.3. PurKine? His-Tag Ni-Super Resin is compatible with a wide range of chemicals: 6M Gua-HCl (Lane D), 1M NaCl (Lane E)
Sweeteners refer to Food Additives that can impart sweetness to soft drinks. Sweeteners can be divided into nutritive sweeteners and non-nutritive sweeteners according to their nutritional value; according to their sweetness, they can be divided into low-sweetness sweeteners and high-sweetness sweeteners; according to their source Divided into natural sweeteners and synthetic sweeteners.
Here you can find the related products in Sweeteners, we are professional manufacturer of Sweeteners. We focused on international export product development, production and sales. We have improved quality control processes of Sweeteners to ensure each export qualified product.
Here you can find the related products in Sweeteners, we are professional manufacturer of Sweeteners like D-Mannose, Raffinose, Sucralose, Maltitol, Erythritol, Sorbitol and so on.
D-Mannose, Raffinose, Sucralose, Maltitol, Erythritol, Sorbitol
Xi'an Gawen Biotechnology Co., Ltd , https://www.ahualyn-bios.com