Prothena terminates the clinical development of amyloidosis lead drug NEOD001
Prothena terminates the clinical development of amyloidosis lead drug NEOD001
April 27, 2018 Source: Sina Pharmaceutical News
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Prothena is a late-stage clinical Irish biotechnology company focused on discovering, developing and commercializing new antibody drugs for the treatment of a variety of diseases associated with protein misfolding or cell adhesion. Recently, the company announced that it will discontinue the clinical development of its lead drug NEOD001, which was developed for AL amyloidosis and has previously failed in the Phase IIb clinical study PRONTO (NCT02632786). Another phase III clinical study VITAL (NCT02312206) It is expected to fail. After the news was released, Prothena's share price plummeted 63% in pre-market trading, falling from $36.84 to $13.69.

In the statement, Prothena acknowledged that NEOD001 failed to reach the primary endpoint and three secondary endpoints in the PRONTO study. The study was a randomized, double-blind, placebo-controlled study of 129 patients with AL amyloidosis who had previously received treatment with persistent cardiac insufficiency. In the study, patients were randomized to receive an intravenous infusion of 24 mg/kg NEOD001 (n=66) or placebo (n=63) every 28 days.
The data showed that after 12 months of treatment, only 39.4% of patients in the NEOD001 treatment group achieved the primary endpoint of optimal cardiac remission (NT-proBNP, a modified consensus criterion) compared with 47.6% in the placebo group. In addition, NEOD001 did not show an advantage or statistically significant improvement in placebo compared to placebo, including: mean change in physiology and health score (PCS) on the SF-36v2 scale (0.19 vs 0.97 [placebo]), medium Changes in the 6-minute walk test distance after 12 months of treatment (19.25 m vs 8.00 m [placebo]), NT-proBNP change rate (slope) during 12 months of treatment (9.80 vs 8.14 [placebo]).
In addition, Prothena has requested the Independent Data Monitoring Committee to review the ineffective analysis of the ongoing Phase III clinical study VITAL (NCT02312206). Based on invalid analysis data, it is recommended to terminate the VITAL study. The study was also a randomized, double-blind, placebo-controlled study in 260 newly diagnosed, newly diagnosed AL amyloidosis patients with cardiac insufficiency. In the study, patients were randomized to receive an intravenous infusion of 24 mg/kg NEOD001 or placebo every 28 days in a 1:1 ratio, and both groups received standard care. The primary endpoint of the study was event-based all-cause death or heart disease hospitalization. Secondary endpoints included SF-36v2-PCS total score, 6-minute walk test distance, and NT-proBNP optimal response.
Based on the failure of the PRONTO study and the expected failure of the VITAL study, Prothena made a decision to terminate all clinical development projects for NEOD001, including VITAL and related open label extension studies.
Dr. Gene Kinney, CEO of Prothena, said that the results from these two placebo-controlled studies have surprised us, and the company will continue to analyze the data and share insights with partners in the science, medicine, and advocacy communities.
NEOD001 is an experimental monoclonal antibody that targets soluble and deposited aggregate amyloids with a specific target for a "concealed" epitope found on the misfolded light chain protein produced by clonal plasma cells. These amyloids accumulate in tissues and organs of patients with AL amyloidosis and may cause organ damage or failure.
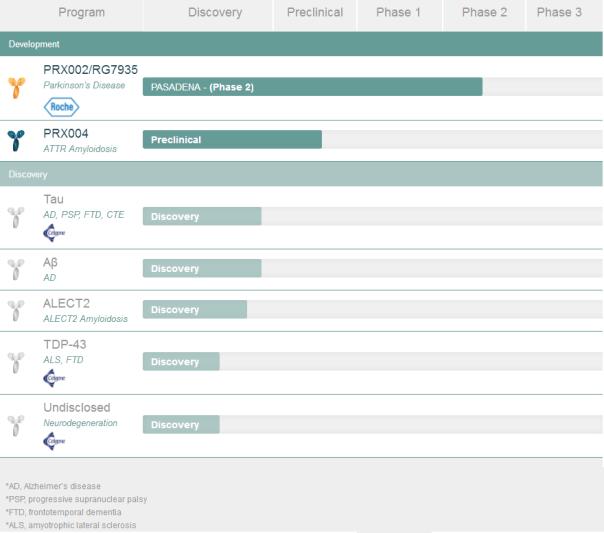
In addition to NEOD001, Prothena has only one clinically developed pipeline drug, PRX002/RG7935, a monoclonal antibody targeting alpha-synuclein, developed in collaboration with Roche and currently in Phase II clinical study PASADENA. (NCE03100149), assessing the potential for treating Parkinson's disease.
Last month, the company and New Base reached a $2 billion cooperation agreement to develop three preclinical assets for the treatment of neurodegenerative diseases. One of the fastest advances was a monoclonal antibody targeting tau, developed for Alzheimer's disease, progressive supranuclear palsy, frontotemporal dementia (FTD), and chronic traumatic encephalopathy. The other two drugs are: TDP-43, developed for amyotrophic lateral sclerosis and FTD; an undisclosed drug candidate developed for a neurodegenerative disease.
Prothena's pipeline assets are shown below: (Sina Pharmaceutical Compilation/newborn)
Article Reference Source: Prothena Scraps Lead Candidate NEOD001 Following Clinical Failures
1, Material: made of medical grade polypropyle(PP) and PS.
2, Capacity: 30ml,40ml,60ml,80ml,90ml,100ml,120ml.
3, Feature: a.With screw cap. The screw cap can be made in any color.
b.Water-proof design can prevent the specimen from leaking out.
4, Usage: The specimen containers are mainly used for urine collection.
5, Color: Red,Blue,Yellow,etc
6, Sterile: E.O. Sterile or Non-Sterile
Urine Container,90Ml Medical Disposable Urine Container,Pp Disposable Urine Sample Container,40Ml Urine Sample Container,60Ml Disposable Urine Container
Changchun ZYF science and technology CO.,LTD , https://www.zyf-medical.com