Summary of recent progress in the field of cancer (02.04)
Summary of recent progress in the field of cancer (02.04)
February 05, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];1. Prevent tumor metastasis! New immunotherapy "vaccine" can clear the same type of tumor
In recent years, immunotherapy has revolutionized cancer treatment, but their preparation is time consuming and expensive, so a research team at Stanford University is exploring ways to stimulate the immune system to recognize and attack cancer cells in a simpler and cheaper way. They have achieved positive results in mouse trials and have recently started clinical trials, which are expected to include approximately 15 lymphoma patients. Related papers are published in Science Translational Medicine.
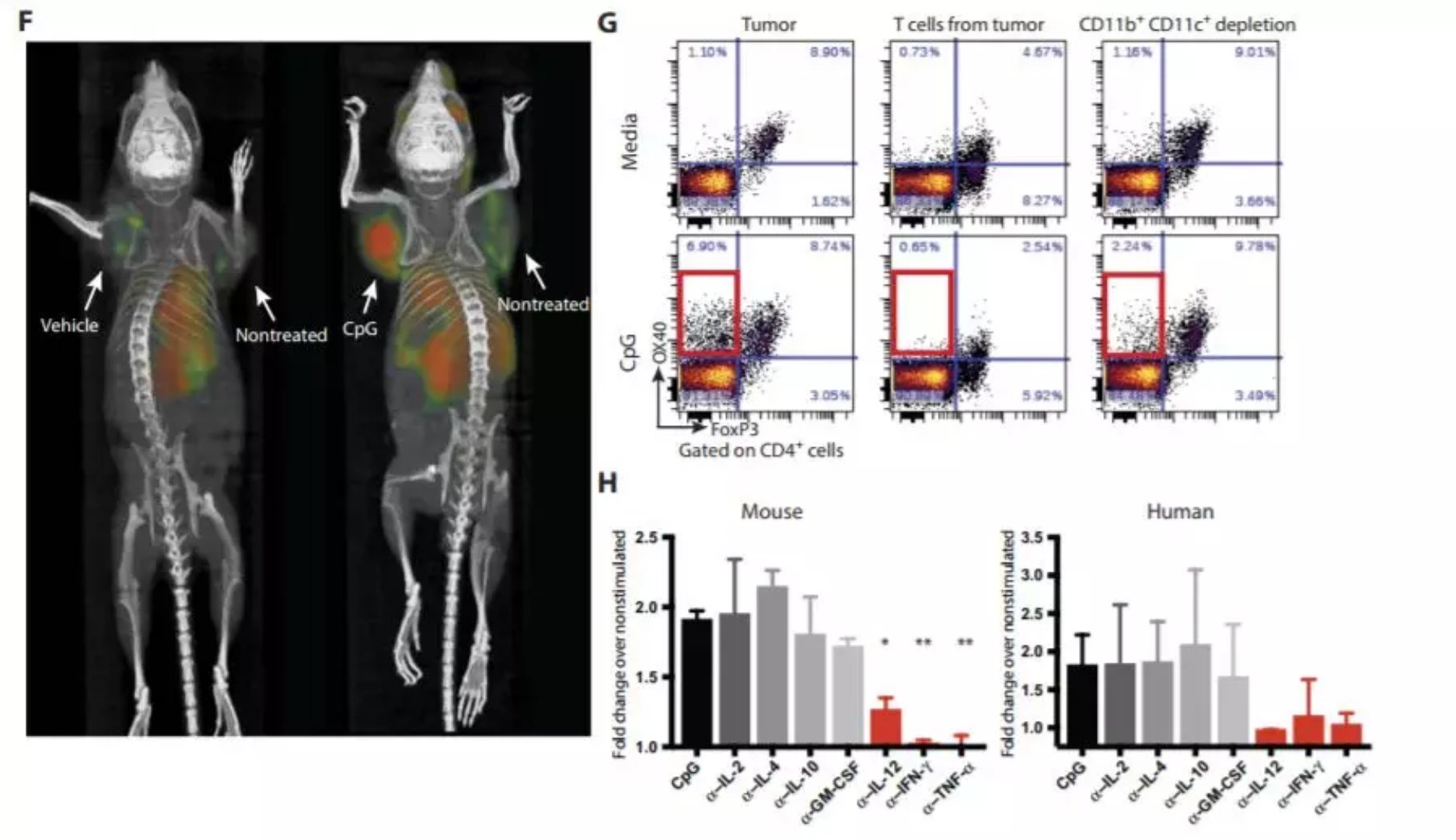
â–²This therapy can induce the expression of OX40 (Source: Science Translational Medicine)
This new immunotherapy is achieved by injecting two molecules that promote the immune response directly into the tumor. One of these molecules is called CpG oligonucleotide (CpG oligonucleotide), which is a small piece of DNA that increases the expression of OX40, a protein expressed on the surface of immune T cells. The other is an antibody that binds to OX40 and directs T cells to eliminate cancer cells. Although this treatment is directed at T cells in tumors that have been injected, the researchers have shown that certain specific immune cells leave the original tumor and move through the body to find and destroy similar tumors. Researchers want to prove that this treatment can direct T cells to recognize specific proteins that are injected into the tumor, even in other parts of the body, so they implant the same type of lymphoma cells in two locations in the mouse. Different types of colon cancer cells were also transplanted as controls. After the injection of one of the lymphomas, the growth of both lymphomas was inhibited, but different types of colon cancer continued to grow without treatment. In the mouse experiment, 90 mice were implanted with lymphoma at two sites, and 87 of the mice disappeared after receiving treatment for one tumor. The other 3 cases also showed tumor disappearance after re-treatment. In addition to lymphoma, this therapy has shown excellent therapeutic effects in mice with breast cancer, colon cancer, and melanoma.
"Our method is to use a small amount of two drugs at a time to stimulate immune cells inside the tumor. We have seen amazing systemic effects in mice that can eliminate tumors in other parts of the animal," said Ronald Levy, Professor of Oncology, Stanford University. "This method does not require the identification of tumor-specific immune targets, and does not require a full activation of the immune system or customizing the patient's personalized immune cells," says Dr. Levy, who believes the treatment has the potential to perform surgery in patients. Injection into the tumor prior to removal of the tumor to prevent tumor cell metastasis from causing recurrence.
2. Positive results of new clinical trials of CAR-T cell therapy
Novartis recently announced the latest results of the key ELIANA clinical trial on Kymriah cell therapy published in the New England Journal of Medicine (NEJM). This clinical trial was conducted in adolescent patients with relapsed or refractory (r/r) B-cell acute lymphoblastic leukemia (ALL).

Kymriah is a novel immunotherapy that uses the patient's own T cells to fight cancer. It uses a 4-1BB costimulatory domain in chimeric antigen receptors to enhance cell expansion and persistence. Kymriah received FDA approval in August 2017 for the treatment of patients with refractory B-cell precursor ALL or a second relapse of disease 25 years of age and younger.
ELIANA (formerly known as CTL019) is the first global clinical trial of CAR-T cell therapy for adolescents in 25 centers in the United States, Canada, Australia, Japan and the European Union. The new data included long-term follow-up (more than three months) and efficacy evaluation of 75 infusion patients, as well as long-term persistence, amplification, and safety analysis of Kymriah. The data showed that the overall response rate was 81% (95% CI: 71%-89%). 60% of patients achieved complete remission (CR), 21% achieved CR with incomplete recovery of blood count (CRi), and no trace residual disease (MRD) was found in all patients (95% on day 28) [58/ 61]).
The median follow-up of these patients was 13.1 months. The median duration of remission has not been reached in patients with CR/CRi. The remission effect was long-lasting, and the relapse-free survival rate at 6 months was 80%. The event-free survival at 6 months was 73% (95% CI: 60%-82%), and at 12 months it was 50% (95% CI: 35%-64%), yet Achieve a median event-free survival period. At 6 months, 75 patients had a total survival of 90% (95% CI: 81%-95%) and 76% at 12 months (95% CI: 63%-86%). At the end of the data analysis, Kymriah had a median duration of 168 days (range: 20-617; 60 patients with CR/CRi) and Kymriah was detected in some patients for up to 20 months. All patients with remission showed B-cell dysplasia (low or even missing B cells), which represents a targeted effect of Kymriah treatment, and most patients received immunoglobulin replacement therapy. In patients who developed remission on day 28, the median time to maximal expansion was 10 days (5.7-28 days; 60 patients), and in 6 patients who did not experience remission, the median time to maximal expansion was 20 days. (13-63 days).
“Kymriah is the first FDA-approved CAR-T cell therapy that demonstrates the potential to be a definitive treatment that provides rapid, deep and long-lasting relief for adolescents with relapsed or refractory ALL,†Novartis Oncology Global Dr. Samit Hirawat of the Drug Development Department commented: "These data demonstrate our commitment to Novartis, which continues to develop CAR-T cell therapy to benefit as many patients as possible."
3. Regardless of the type of tumor! The initial clinical trial results of the new kinase inhibitors are gratifying
Puma Biotechnology recently announced that the company's ongoing phase 2 clinical trial "SUMMIT" preliminary results were published in the journal Nature, which was designed to evaluate the efficacy of PB272 (neratinib) in patients with HER2 or HER3 mutations. Neratinib is a dual kinase inhibitor against HER2 and EGFR.
SUMMIT is a multi-histological, open-label, global study evaluating the safety and efficacy of neratinib daily in a variety of solid tumor patients with activating HER2 or HER3 mutations. Preliminary results included data from 141 patients in the neratinib-treated group, including 124 patients with HER2 mutations and 17 patients with HER3 mutations. The investigators observed 30 different HER2 mutations and 12 different HER3 mutations in these patients. . The study covered 21 unique tumor types, the most common being breast, lung, bladder and colorectal cancer.
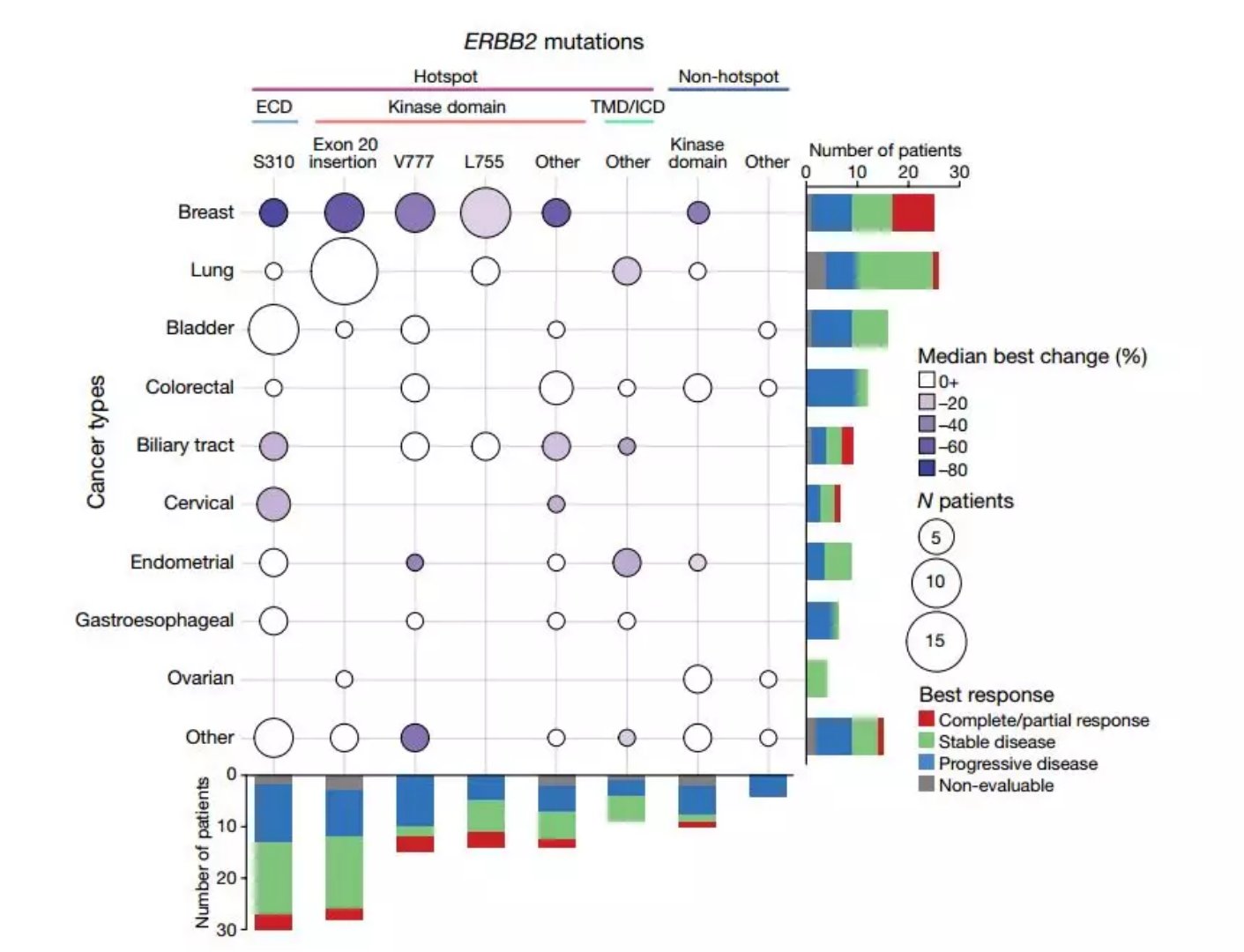
â–²The efficacy of different tumor types and different mutations are different (Source: Nature)
The effect of Neratinib was genotyped, and clinical responses occurred in tumors of the S310, L755, V777, P780_Y781insGSP and A775_G776insYVMA mutants of HER2. No activity was observed in the HER3 mutant population. When viewed from the tumor type, a response was observed in patients with breast cancer, cervical cancer, cholangiocarcinoma, salivary gland cancer, and non-small cell lung cancer.
“SUMMIT's initial data can be published in the top journal Nature, reflecting the novelty and quality of this drug trial design, and recognizing that tumor types and gene mutations play an important role in patients' response to cancer treatment, Alan H. Auerbach, Puma's CEO and President, said: "The experimental design used by SUMMIT allows researchers to assess the clinical potential of neratinib in a variety of cancer types, rather than being limited to one type of tumor at a time. SUMMIT is significant because it will provide the largest clinical data to date using irreversible multiplexed HER inhibitors in solid tumor patients with somatic HER2 or HER3 mutations."
4. Lee's large pharmaceutical company PD-L1 monoclonal antibody obtained CFDA clinical approval
Lee's Pharm (Li's Pharmaceuticals) and Sorrento Therapeutics recently announced that China Cancer Medical Co., Ltd. (COF, a subsidiary of Lee's Pharmaceuticals) has officially obtained the national anti-PD-L1 monoclonal antibody injection ZKAB001. Clinical approval of the Food and Drug Administration (CFDA). The development of this antibody cell line in Greater China is exclusively authorized by Sorrento Therapeutics.

ZKAB001 clinical trial units include: Beijing Cancer Hospital, Cancer Hospital of Chinese Academy of Medical Sciences, Wuhan Union Hospital and the Sixth People's Hospital affiliated to Shanghai Jiaotong University. This clinical trial will use a 3+3 design of 5 mg/kg, 10 mg/kg and 15 mg/kg dosing regimens. Once the maximum tolerated dose (MTD) is determined, the number of cases will be increased in the expanded clinical phase 1 protocol. It is expected that clinical data will be obtained by the end of 2019. Such data can reasonably predict or judge its clinical benefit and has obvious advantages over existing treatments. It can be approved by the CFDA for conditional approval before the completion of the phase 3 confirmatory clinical trial.
ZKAB001 is a fully human PD-L1 monoclonal antibody directed against tumor immunological checkpoints. The antibody binds to PD-L1 protein and blocks the interaction between PD-L1 protein and its receptor PD-1, thereby inhibiting the inhibition of T cells by PD-1 or PD-L1 signaling pathway and enhancing the killing of tumor by T cells. effect. The antibody is also capable of killing cancer cells by conventional antibody-dependent cell-mediated cytotoxicity (ADCC). The antibody variable region (Fv) binds to PD-L1 on cancer cells, and the antigen crystallized region (Fc) binds to immune cells such as NK cells, enabling immune cells to directly kill cancer cells and further enhance antibody anti-cancer. effect.
“We are pleased to announce that our anti-PD-L1 antibody ZKAB001 has been approved for clinical research (IND), which further demonstrates that COF continues to be committed to meeting the unmet need to bring new and effective immuno-oncology therapy to the Chinese market. Oncology needs. The COF team worked closely with Sorrento colleagues throughout the IND initiation and review process. In the field of anti-PD-L1 treatment in China, we believe that our project is part of the first wave of immunological checkpoint inhibitors. Encouraged by impressive clinical data, we are excited to evaluate immunotherapy and address the unmet needs of cancer patients in Greater China. Based on the efficient collaboration of our first project, we look forward to expanding our area of ​​cooperation with Sorrento. "COF CEO and Executive Director Dr. Li Xiaoyu said.
5. The anti-cancer means that I am looking for is always around us.
Oncolytic virus is an emerging therapy in the field of cancer therapy. These viruses are capable of specifically infecting tumor cells and replicating proliferation therein depending on the difference in immunity between tumor cells and healthy cells. When the new virus breaks through the cell and spreads further, it is the death of the tumor cell. However, many patients develop resistance to this viral therapy. For example, oncolytic viruses based on Vesicular Stomatitis Virus (VSV) are only effective in one-third of cancers. The reason is that the immune system of tumor cells produces an anti-viral immune response when the virus invades. These immune responses usually inhibit further proliferation and spread of the virus. Therefore, the use of other drugs that inhibit the immune response in combination with oncolytic viruses may have miraculous effects.

â–²This study was published on the cover of Science Translational Medicine (Source: Science Translational Medicine)
Scientists at the Ottawa Hospital Research Institute in Canada have discovered that Biogen's treatment of multiple sclerosis (MS) in Tecfidera can improve the anticancer effect of oncolytic viruses. Its main ingredients It is Dimethyl Fumarate (DMF). MS is an autoimmune disease, and DMF is able to inhibit inflammatory responses and protect nerves, so it is approved by the FDA for the treatment of MS.
The researchers found that using DMF 4 hours before tumor cells received VSV infection increased the number of new VSVs by a factor of 100! Further research indicates that this is because the virus can infect more tumor cells. This effect can be achieved in a variety of cancer cell lines including sarcoma, osteosarcoma, colon cancer, breast cancer, melanoma and ovarian cancer. The role of DMF in promoting viral infection and transmission is not limited to VSV, it can also increase the spread of Sindbis virus, herpes virus, and adenovirus in tumor cells.

In experiments with tumor explant models, xenograft mouse models, and syngeneic tumor mouse models, the researchers found that DMF can not only improve the spread of oncolytic virus in tumor tissues, but also Increasing the anticancer effect of oncolytic viruses significantly delays tumor proliferation. Even in some mouse models, this combination therapy can lead to complete tumor remission in 20% of mice. When analyzing the gene expression profile of tumors by gene chip, the researchers found that when the oncolytic virus infects tumor cells, the tumor cells increase the expression of various antiviral genes, including many cytokines. DMF is able to inhibit the expression of most antiviral genes. The type I interferon (IFN)-activated signaling pathway plays an important role in the antiviral immune response of cells. DMF can reduce the expression of IFN by regulating NF-κB and STAT1 signaling pathway, and inhibit the response of cells to IFN-induced signaling pathway, thus reducing the resistance of tumor cells to oncolytic virus.
Since its approval by the FDA for the treatment of MS, Tecfidera has been used by more than 135,000 patients worldwide, so its safety has been well documented. And the first oncolytic virus-based anticancer therapy has also received FDA approval. Canadian researchers' new discovery of anti-cancer effects of combination therapy with DMF and oncolytic virus means that from a clinical development perspective, researchers have a clear path to test this combination therapy in human patients through clinical trials. Efficacy.
Reference material
[1] Tumor 'vaccine' clears several cancer types in mice
[2] Novartis Announces NEJM Publication of Updated Analysis From ELIANA Trial Showing Longer-Term Durable Remissions With Kymriah in Children, Young Adults With r/r ALL
[3] Puma Biotechnology Announces Publication of Results from Phase II SUMMIT 'Basket' Trial Evaluating Neratinib in HER2 and HER3 Mutant Cancers
[4] China Oncology Focus Limited Receives Approval by Chinese Authorities to Begin Clinical Trials in Three Separate Cancer Indications Using Sorrento's Anti-PD-L1 Monoclonal Antibody
[5] Biogen's MS drug Tecfidera could make cancer-killing viruses more potent: study
Original title: Summary of recent progress in the field of cancer (No. 53)
I. Company profile
Tiancheng Chemical Co., Ltd. is a collectivized high-tech multi-function fine chemical enterprise, integrated with science, industry, trade and research, which is mainly engaged in paper chemicals, Water Treatment Chemicals, rubber and plastic chemicals, agricultural pharmaceutical intermediates, basic chemical materials, food additives, detergent additives and electronic chemicals.
II. Technical indicators:
|
Appearance |
Colorless and transparent liquid |
|
Content |
60% |
|
pH value |
4-5.5 |
|
Viscosity (25℃), mPa·s |
≤20 |
III. Application fields:
1. In the filed of cosmetics, detergents, wool detergents: With the continuous improvement of people`s living standards, the demand for cosmetics, washing products and cleaning products is continuously increasing. As a monomer, the surfactant is applied to many fields, but also use with non-ion preparations jointly, with the function of penetration, humidification, washing and electrostatic elimination, providing help and support for improvement of people`s life and quality of life.
2. In the field of electronic chemicals: the surfactant can be used in cleaning preparations of the electronic products, with the function of washing and electrostatic elimination.
IV. Notes:
1. Storage condition: This product is stored in a cool and dry place (5-30 ℃) to prevent open-air exposure and freezing.
2. If the product storage temperature is below -15 ℃, there will be crystalline polamer. Before using, please heat and stir evenly, not affecting the product quality.
3. The product is slightly acidic. If it is accidentally into the eyes, please wash with water immediately.
V. Packing specification
250 kg plastic drum; IBC tons of barrel; Flexitank.

Surface Active Agent Tc-1000,Surface Active Agent,Surface Active Agent For Electronic Chemicals
Shandong Tiancheng Chemical Co., Ltd. , https://www.tianchengchemical.com