Opening a new perspective of aquatic science research | Succinylation modification group + metabolome reveals the metabolic regulation mechanism of aquatic animal pathogens
In this issue, Xiaobian will bring you an article published in the 19-year-old authoritative journal Molecular & Cellular Proteomics, which uses succinylated modification and metabolome to reveal the succinylation of aquatic pathogens. A key role in quorum sensing and metabolism.


Integrated Succinylome Profiling and Metabolomics Reveal Crucial
Role of S-ribosylhomocysteine ​​lyase
In Quorum Sensing and Metabolism of Aeromonas hydrophila
Molecular & Cellular Proteomics
IF= 5.236
Original link:
Https://
Research Background:
Aeromonas hydrophila is one of the most common pathogens in aquaculture and can cause hemorrhagic sepsis in fish. Aeromonas hydrophila belongs to Gram-negative bacteria, which can monitor the pathogenicity of bacteria in the environment by quorum sensing (QS) and induced signal molecules (Autosducers, AIs). The study found that the activity of S-ribosylhomocysteinelyase (LuxS) plays an important role in quorum sensing, biofilm formation, bioluminescence, toxicity and antibiotic resistance, but post-translational modification The regulatory mechanism of this protease has not been reported.
material:
Aeromonas hydrophila ATGCC7966; control strain (CK) and LuxS enzyme mutant strains (K23R, K30R, K23E, K30E).
Technical method:
Succinylation modification group, metabolomics
Technical route:
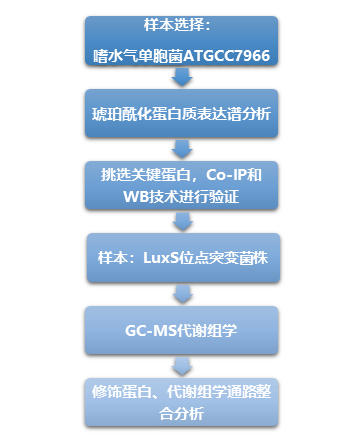
Experimental results:
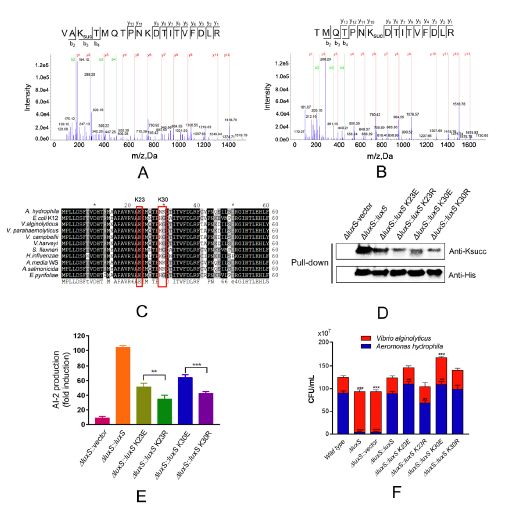
1. Analysis of lysine succinylation modification
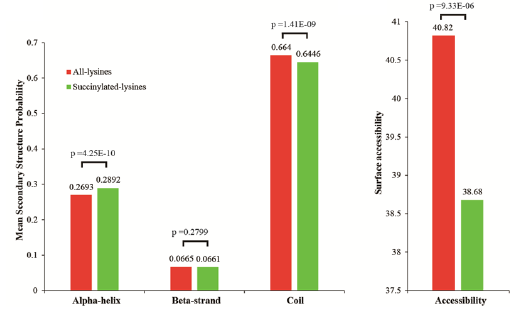
Lysine succinylation modification analysis was performed on Aeromonas hydrophila ATGCC7966. A total of 2174 lysine succinylation sites derived from 666 proteins were finally identified. Further, through the motif analysis, eight conserved motifs were obtained. The lysine succinylation sequence motif analysis showed that different strains had a common preference for the substrate. Protein secondary structure predictions suggest that lysine succinylation may affect protein activity through secondary structural transformation, for example, a lysine site located inside the protein structure is involved in substrate enzyme binding and catalytic activity.
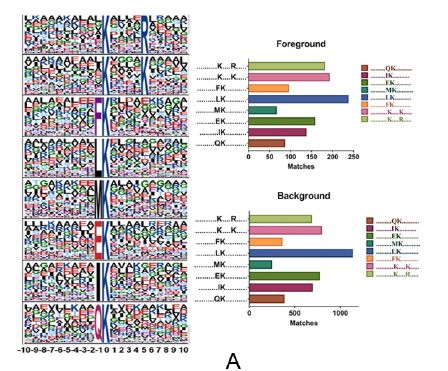
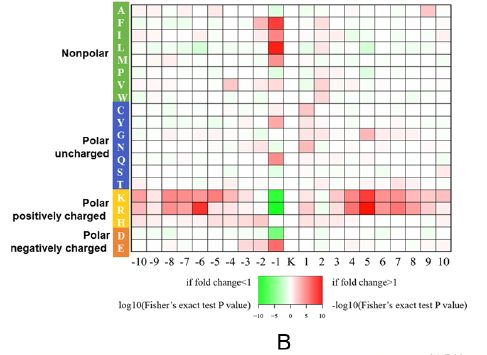
Subcellular localization, biological function and pathway annotation results show that lysine succinylation modified protein mainly exists in cytoplasm, mainly involved in important functions such as core energy metabolism, biosynthesis, translation process and substrate binding. Protein interaction analysis showed that the lysine succinate protein of Aeromonas hydrophila is involved in various metabolic pathways, such as: TCA cycle, pentose phosphate pathway, glycolysis, gluconeogenesis, pyruvate metabolism and the like.
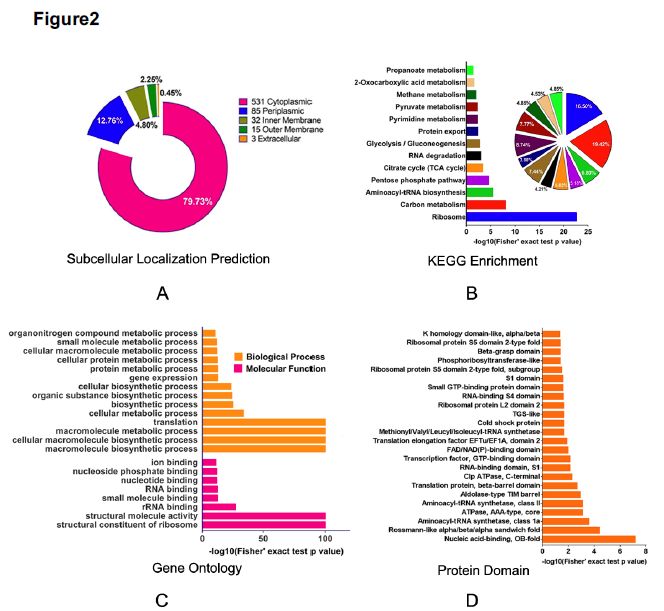
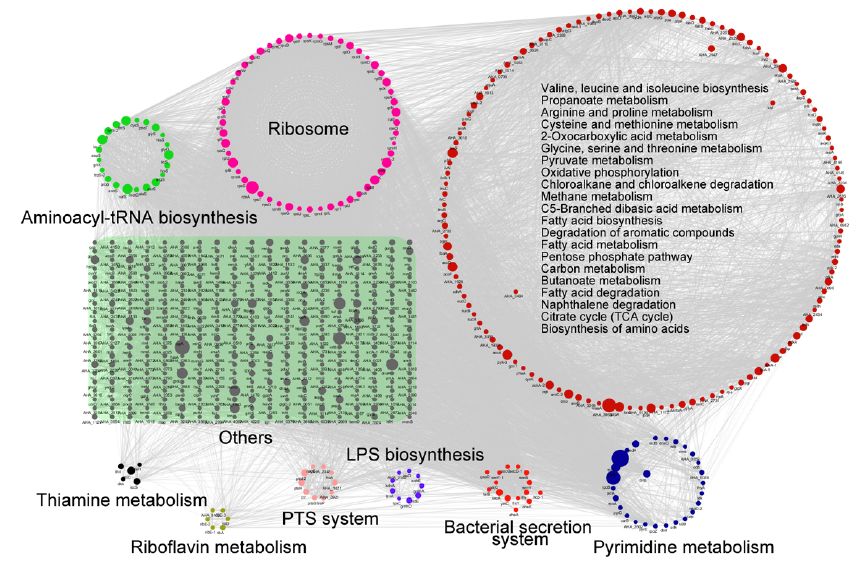
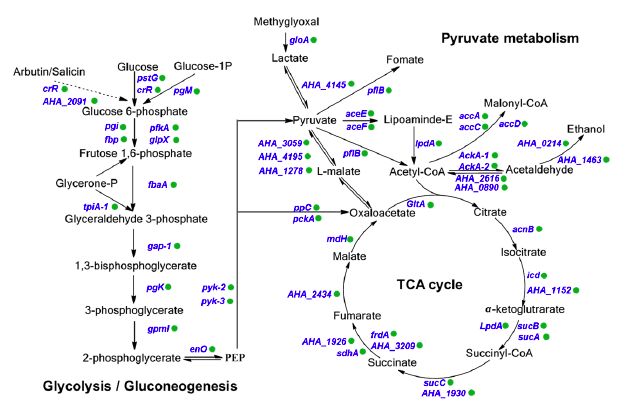
2. Screen the modified protein for verification and explore the function of the modification site
Further, the authors verified six candidate succinylated proteins (LuxS, PkG, etc.), first enriched candidate modified proteins by Co-IP, and then used WBB with succinylated antibodies, demonstrating that these proteins did indeed form succinyl Modification. Subsequently, the authors locked in a synthetase that regulates the quorum sensing autoinducer AI-2, LuxS, and further studied the succinylation of LuxS by mutation of the modified sites K23R and K30R of LuxS. The activity of the protease is being regulated, thereby promoting the synthesis of AI-2 and its communication with other bacteria.
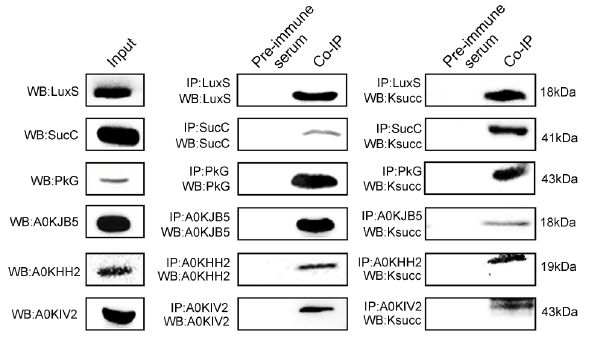
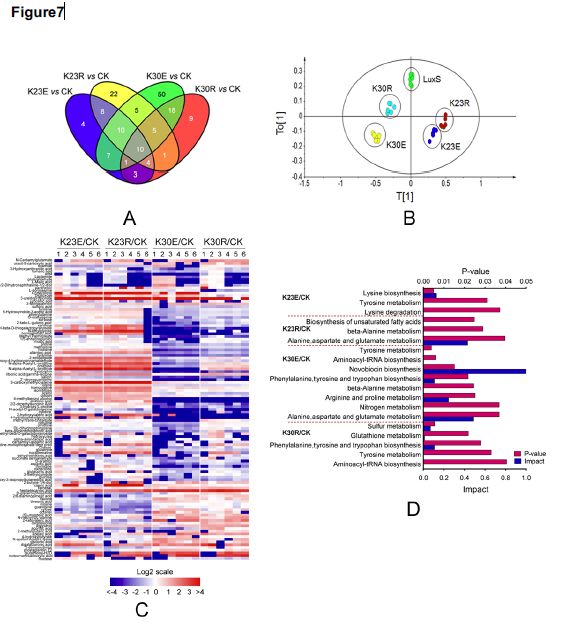
3. Metabolic analysis of LuxS site mutant strain
The authors further performed a metabolome analysis of the LuxS site mutant strain, and a total of 271 metabolites were detected. The results of histology showed that LuxS protease affects amino acid, fatty acid and sulfur metabolism. LuxS subtly regulates the succinylation state of K23 and K30 sites, and subtly controls central metabolic pathways such as amino acid, fatty acid and sulfur metabolism.
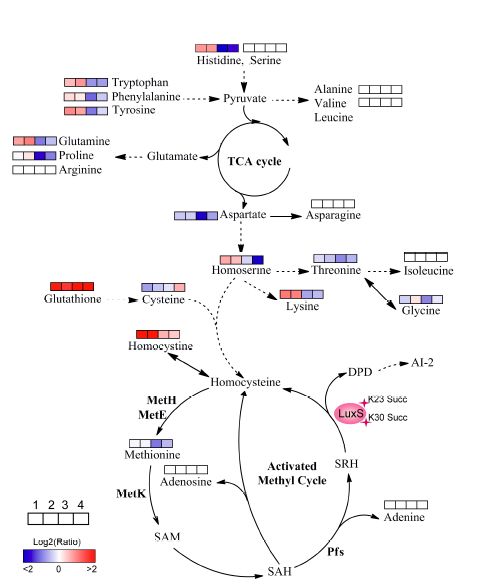
Xiaobian summary:
The authors used the succinylation modification group for analysis and screening in order to find further proteins for further analysis. Due to the extensive regulation of metabolism by acylation modification, the authors finally targeted the S-ribose homocysteine ​​lyase (LuxS), which first demonstrated the effect of the modification site on the phenotype on the enzyme. . To further reveal its more potential functions, the authors performed metabolomics and found that K30 succinylation on LuxS may inhibit the luxs gene in activating the methyl cycle (amc), and both k23 and k30 sites are involved in amino acid metabolism. . Finally, the study revealed that the lysine succinate modification of the LuxS enzyme regulates the growth of bacteria and its adaptation to the dynamic environment of the environment.
This article is an article with simple and clear ideas and smooth logic . It is worth learning that after completing the modified group, locking the target protein can further analyze the metabolome, which is also a good way to improve the quality of the article. At present, Zhongke New Life has a professional technical platform guarantee in terms of modified omics or metabolomics, and has many high-level client literatures. Welcome interested teachers to come to consult.
nitrile glove,latex glove,PVC glove,single pair packed latex glove,rubber glove
Shandong Zhushi Pharmaceutical Group Co.,LTD , https://www.sdzs-medical.com