Reata's Bardoxolone for the treatment of two rare kidney diseases in phase II clinically positive data
Reata's Bardoxolone for the treatment of two rare kidney diseases in phase II clinically positive data
July 24, 2018 Source: Sina Pharmaceutical
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Reata Pharma is a clinical-stage biopharmaceutical company dedicated to developing innovative therapies for the treatment of serious or life-threatening diseases through molecular pathways involving cellular metabolism and inflammatory regulation. Recently, the company published data from two Phase II clinical studies of the experimental drug bardoxolone for chronic kidney disease (CKD), including one-year data from the Phase II study of the CARDINAL project in patients with CKD caused by Alport syndrome. And the final data of the phase II clinical study PHOENIX autosomal dominant polycystic kidney disease (ADPKD) cohort.

- 1-year data from the Phase II study of the CARDINAL program showed that at week 48, the estimated glomerular filtration rate (eGFR) was estimated relative to baseline (n=25) in patients treated with bardoxolone (n=22). Significant increase in meaning (increase: 10.4 mL / min / 1.73 m2, p < 0.0001). The historical eGFR data from 22 of the 25 patients in the study showed that the renal function of these patients decreased at a rate of 4.2 mL/min/1.73 square meters per year before entering the study. One year after receiving bardoxolone treatment, an improvement in eGFR of 10.4 mL/min/1.73 square meters was observed to represent a recovery of mean eGFR loss of approximately 2 years.
After 4 weeks of discontinuation, at week 52, the patient's eGFR still achieved a significant increase relative to baseline (increase: 4.1 mL/min/1.73 m2, p<0.05). These results provide evidence that bardoxolone can delay or prevent renal failure. The FDA has provided guidance to Reata, Inc., in patients with Alport syndrome, if placebo-corrected eGFR achieves significant improvement after one year of bardoxolone treatment, it can support accelerated approval of bardoxolone; if bardoxolone is treated 2 years later via placebo correction The eGFR also achieves significant improvements, which can support full approval.
In this study, treatment did not report serious adverse events associated with treatment, and all adverse events were mild to moderate. Safety analysis was performed in 25 patients and no treatment interruption occurred.
- Final data from the PHOENIX study ADPKD cohort showed a significant statistically significant increase in eGFR relative to baseline at bardoxolone treatment at week 12 (increase: 9.3 mL/min/1.73 m2, p<0.0001), reached The primary endpoint of the study. The historical eGFR data from 29 of the 31 patients in the study collected showed that their renal function decreased at a rate of 4.8 mL/min/1.73 m2 per year before entering the study. After 12 weeks of treatment with bardoxolone, the observed improvement of 9.3 mL/min/1.73 square meters represents a recovery of mean eGFR loss of approximately 2 years.
In this study, safety data from the ADPKD cohort showed no serious adverse events associated with treatment, and all adverse events were mild to moderate. Safety analysis was performed in 28 patients, and only 1 patient (3%) discontinued treatment for treatment-related fatigue events.
Warren Huff, president and CEO of Reata, said the results have further enhanced clinical evidence that bardoxolone has the potential to prevent or delay kidney failure in a rare class of CKD. Importantly, data from CARDINAL showed that in patients with Alport syndrome who received standard care but whose renal function was still declining, the drug treatment significantly improved kidney function in one year. In addition, the benefit of eGFR relative to historical eGFR observed after 4 weeks of bardoxolone discontinuation suggests that the retained eGFR benefit in the CARDINAL Phase III study is somewhat conservative. In addition, 12-week treatment data from patients with ADPKD showed that long-term improvement data for other types of CKD in this drug is expected to translate into therapeutic benefit for patients with ADPKD.
Bardoxolone is an experimental, oral, once-a-day Nrf2 activator, a transcription factor that induces molecular pathways that restore mitochondrial function, reduce oxidative stress, and inhibit pro-inflammatory signaling to promote regression of inflammation. In the United States and the European Union, FDA and EMA have granted bardoxolone the orphan drug qualification for Alport syndrome; in addition, the FDA has granted bardoxolone the orphan drug qualification for pulmonary hypertension (PAH).
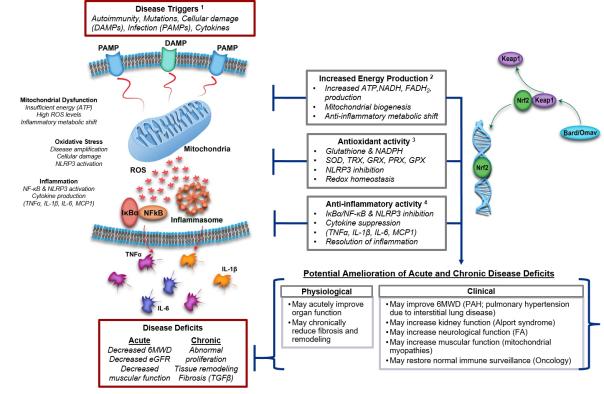
Mechanism of action of Nrf2 activator (from Reata's official website)
In addition to the CARDINAL and PHOENIX studies, Reata is also conducting two additional Phase III studies, CATALYST and AYAME, to evaluate the efficacy and safety of bardoxolone in the treatment of connective tissue disease-associated pulmonary hypertension and diabetic nephropathy. (Sina Pharmaceutical Compilation/newborn)
Article Reference Source: Reata Announces Positive Phase 2 Data for Bardoxolone Methyl in CKD Caused by Alport Syndrome and Autosomal Dominant Polycystic Kidney Disease
Active Pharmaceutical Ingredients
Active Pharmaceutical Ingredients(API) refer to the raw materials used in the production of various preparations. They are the effective ingredients in the preparations. They are various powders, crystals, extracts, etc., prepared by chemical synthesis, plant extraction or biotechnology, but Substances that the patient cannot take directly. API is intended to be used in any substance or mixture of substances in the manufacture of pharmaceuticals, and when used in pharmaceuticals, it becomes an active ingredient of the pharmaceuticals. Such substances have pharmacological activity or other direct effects in the diagnosis, treatment, symptom relief, treatment or prevention of diseases, or can affect the function or structure of the body. According to its source, active pharmaceutical ingredients are divided into two categories: synthetic chemical active Pharmaceutical ingredients and natural chemical active Pharmaceutical ingredients.
Chromium Picolinate,Tianeptine,6-Paradol,Aminobutyric acid,acetylcysteine,L-Carnosine
Xi'an Gawen Biotechnology Co., Ltd , https://www.ahualynbios.com