Advances in studies on the interaction mechanism of NK cell-activated receptor molecule DNAM-1 and its ligands
Advances in studies on the interaction mechanism of NK cell-activated receptor molecule DNAM-1 and its ligands
January 04, 2019 Source: Institute of Microbiology, Beijing Institute of Life Sciences
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Recently, the Proceedings of the National Academy of Sciences (PNAS) reported the research results of the interaction mechanism between DNAM-1 and ligand CD155 by the Microbiology Institute of the Chinese Academy of Sciences and the Gaofu team of the Beijing Institute of Biological Sciences of the Chinese Academy of Sciences.
The steady state of the human body environment is a necessary condition for the body to carry out normal life activities, and the body's immune system is an important regulation mechanism to maintain the body's homeostasis. In the immune system, natural killer (NK) cells are a subset of lymphocytes that are differentiated from hematopoietic stem cells and are a natural force for natural and cellular immunity. It can release perforin and granzyme directly to mediate necrosis and apoptosis of target cells, and also regulate various immune cell functions such as T and B lymphocytes by releasing various cytokines, and finally achieve the purpose of immune surveillance and immune homeostasis. .
The function of NK cells is controlled by the coordination of two types of receptor molecules, “yin†and “yang†on the cell surface. Paired inhibitory and activated receptors are capable of recognizing the same MHC class I molecule. Since the affinity of the inhibitory receptor to the ligand is high, the NK cell is usually in a suppressed state. Stimulating signals such as viral infections lead to overexpression of activated receptors, competitive binding of ligands, activation of NK cells, "killing" of pathogenic microorganisms entering the body or removal of self-existing diseased cells in the body.
In recent years, a new class of newly discovered paired NK cell receptor families has gradually entered the line of sight. This is the TIGIT-CD226-CD96 consisting of the inhibitory receptors TIGIT, CD96 and the activated receptor DNAM-1 (CD226). Recipient family. The receptor family molecules regulate NK cell function by specifically recognizing the same ligand, the nectin/Necl family molecule CD155 (Necl-5). As an inhibitory immunological checkpoint molecule, TIGIT is widely used in the research and clinical application of various target cell therapy. DNAM-1 is the only activated receptor molecule in this family. Therefore, studies on the mechanism of action of DNAM-1 and its ligands can not only elucidate the mechanism of action of activating NK cells, but also have important value in the development of immunotherapeutics targeting DNAM-1.
The Gaofu team analyzed the high-resolution three-dimensional structure of the extracellular segment of human DNAM-1 and mouse DNAM-1 molecules. In general, proteins with multiple Ig-like domains typically form a "head-to-tail" tandem approach between the Ig-like domains (see CD155 molecules). This "bead"-like arrangement results in some flexibility between adjacent two Ig domains, allowing them to bind receptor/ligand molecules through conformational adjustment. The researchers found that the two MgV domains, composed of two V-type immunoglobulin (IgV) domains, exhibit a unique "side-by-side" side-by-side arrangement. This special tandem approach allows the beta sheets of the two domains to form a "rigid" monolith through a hydrogen bond network, resulting in a "super-Ig" structure. area. A similar arrangement pattern is found only in the structure of the variable-region containing chitin-binding protein 3 (MVBP3). The researchers speculate that the "shoulder-to-shoulder" rigid arrangement between the Ig domains may have an effect on their function, and this particular arrangement provides insight into the evolution of ancient immune molecules such as immunoglobulins. New clues.
The team also systematically studied the binding mechanism of DNAM-1 molecule to its ligand CD155 molecule. By analyzing the structure of the DNAM-1/CD155 complex, they found that DNAM-1 mainly passes the IgV (CD226-D1) domain at the distal end and the IgV (CD155-D1) domain at the far end of CD155. The combination mode of (double-lock-and-key)" is combined. Then, by detecting the binding ability of the truncated protein and mutant protein of different domains of DNAM-1 to CD155, they found that the near-membrane IgV (CD226-D2) domain of DNAM-1 not only maintains the overall conformational stability of CD226 molecule. It plays an important role in sex and is more directly involved in the interaction with CD155 molecules. This particular mode of binding has never been seen in the binding of TIGIT-CD226-CD96 family molecules to ligands.
The researchers also found that the binding receptor DNAM-1 and the paired inhibitory receptor TIGIT are at the same level of binding ability to the ligand CD155. This result suggests that the regulation of NK cell function by the TIGIT-CD226-CD96 family may be different from the previously known "suppression signal-first" regulatory pattern.
In summary, the clarification of the mode of action of DNAM-1 and its ligands is important for understanding the mechanism of action of TIGIT-CD226-CD96 family receptor molecules and ligands and the regulation mechanism of NK cells. At the same time, the study provides an important theoretical basis for the development of tumor immunotherapeutic antibodies targeting DNAM-1.
In recent years, the research team has carried out a series of work on the mechanism of T cell and NK cell key receptor/ligand mechanism and the mechanism of antibody drug action in tumor immunological checkpoints, in nectin/Necl family molecules and viral surface antigens and immune related receptor molecules. Research on interaction mechanisms has been published in the journals Nature Structure and Molecular Biology, PLoS Pathogens, Structure, Journal of Immunology, and Journal of Virology to understand the mechanism of action and drug development of such molecules in immune regulation and pathogen invasion. Provides an important foundation.
Wang Han, a postdoctoral fellow at the Beijing Institute of Bioscience, is the first author of the paper. Tan Shuguang, an associate researcher at the Institute of Microbiology and Microbiology, is the co-author of the paper. The research was supported by the National Natural Science Foundation, the Chinese Academy of Sciences' Strategic Pilot Science and Technology Specialist, and the Postdoctoral Innovation Talent Support Program.
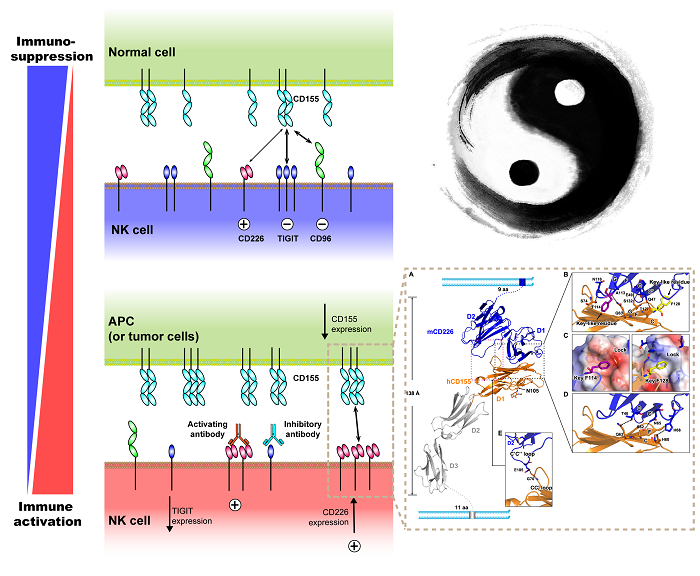
Figure: Interaction of DNAM-1/CD226 with ligand CD155 plays an important role in the activation of NK cells.
Yanfang nitrile gloves have excellent strength and elasticity,we are not only certified with ISO9001, ISO13485 but also fully complied with the essential USFDA, CE Compliances, as well as obtaining relevant accreditation of FDA510K, EN455, and EN374.
disposable nitrile gloves,medical examination gloves,blue nitrile glove,powder free nitrile gloves,medical gloves
Jiangsu Yanfang Medical Technology Co.,Ltd. , https://www.yanfangchina.com