Medtronic fought this track and competed with Google and Apple
A few days ago, Medtronic acquired a long-term partner, AI-assisted nutrition management platform Nutrino. This is not the first time that Medtronic has introduced AI into diabetes management. Through this small sputum, you can see the undercurrent of Medtronic's layout. After that, Medtronic also cooperated with IBM Watson to launch the intelligent diabetes life management software Sugar. IQ.
In addition, Medtronic's competitor Dexcom recently acquired TypeZero, which is also a diabetes management platform and is excellent in insulin delivery algorithms.
They took the same action, it is no coincidence that in the track of diabetes management, the arterial network found that Internet companies such as pharmaceutical companies and Google, Apple, Tencent and other Internet giants are betting on bets.
In this paper, the arterial network attempts to reveal:
1. What kind of logic does Medtronic choose to manage in diabetes management;
2. How do Google, Apple and other giants choose to cut in the "anchor point";
3. What is the development environment of the health management market in the United States;
4. The situation in the domestic market and the opportunities.
Medtronic bets diabetes
In people's minds, Medtronic is a professional player in medical devices. But from the picture below, we can find that Medtronic has completed the comprehensive layout in the field of diabetes, and has corresponding products in various aspects such as prevention, treatment and prognosis.
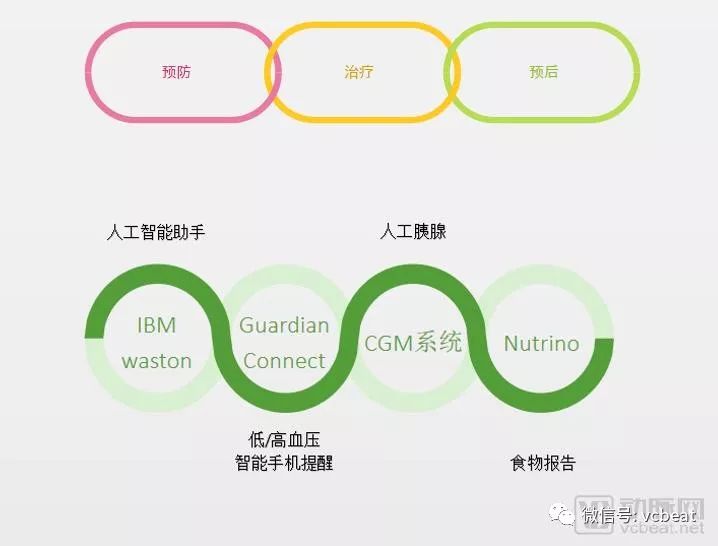
Medtronic Diabetes Digital Health Program Arterial Network Mapping
“Today Medtronic maintains leadership in most of the fastest growing medical technology markets, and we are investing more in faster growth markets and new opportunities.†Medtronic President Omar Ishrak says we are looking beyond simple To improve and innovate existing products and treatments, our goal is to disrupt the market. â€
From this passage, it is enough to see Medtronic's idea: to really solve the medical problem, not just to supplement it, to be a supporting role.
In this regard, Medtronic can be said to be doing a good job. Medtronic's Medtronic MiniMed is the first continuous blood glucose monitoring system approved by the FDA, also known as the “artificial pancreasâ€. MiniMed directly “talks†to the insulin pump by using the Continuous Glucose Monitoring System (CGMS). Under the guidance of the patient, timely injection of the right amount of insulin. This system achieves closed-loop insulin infusion, significantly improving glycemic control, improving patient quality of life, reducing complications, and reducing medical costs.
The FDA is also optimistic about this "artificial pancreas". At the end of June this year, it announced the expansion of the use of MiniMed670G, expanding the use of children to children over 7 years old.
"Artificial pancreas" is more like a technical breakthrough. Let us look at a series of layouts of Medtronic around the "artificial pancreas". It can be seen that Medtronic wants to completely control the healthy rhythm of diabetic patients, rather than being a supporting device.
Then, Medtronic developed the Guardian Connect system that connects the smartphone to the CGM (continuous blood glucose monitor), which was also approved by the FDA. The Guardian Connect system consists of Guardian sensors and connected transmitters that send continuously collected data via Bluetooth to the Guardian Connect APP on the user's smartphone. This app can alert patients 60 minutes before the onset of hyperglycemia or hypoglycemia.
The acquisition of Nutrino, the AI ​​nutrition management platform by Medtronic, is also preparing to integrate Nutrino's predictive blood glucose response algorithm into its technology. In June of this year, Medtronic had reached an agreement with Nutrino to integrate Nutrino's FoodPrint reporting technology into Medtronic's iPro2 myLog application. This technology allows users to see what they want to eat and what they will have for blood sugar. Impact.
In this respect, Medtronic is not a slap in the face, but completely breaks through the last mile of data analysis, and finally provides users with professional intelligence analysis.
Previously, Medtronic and IBM Wston teamed up to launch a diabetes health management software: Sugar. IQ. Currently, the app can be used with the Medtronic Guardian Connect system to form a continuous blood glucose monitoring system. Sugar. IQ is available for iOS-based phones in the United States.
Sugar. IQ is like applying Alexa and Siri to the health management of people with diabetes. Huzefa Neemuchwala, head of global digital health solutions and artificial intelligence at Medtronic, said: "It's a smart assistant that keeps track of all your information and intelligently aggregates all of your information, and then through Watson technology, we can provide insights Suggestions so we can better manage your diabetes."
It is worth mentioning that Medtronic and IBM Watson Health and the cooperation are also approved by the FDA. The FDA approves the use of IBM Watson Health's artificial intelligence analysis to tell users the data and make recommendations.
Why do you need so much data, Huzefa Neemuchwala, senior director of data and information innovation at Medtronic, said: “Diabetes people want to know directly, 'How much insulin do I need now? But this is a question that the FDA will not answer directly because it depends on a lot. Did you sleep last night? Are you ill? Are you stressed this morning? What is your blood sugar? What is your historical blood sugar? There are many factors that affect how much insulin you take now. But if you bring With more and more of these data streams, you can take so much insulin by saying that in the past, similar situations, this is your answer." Data can get us close to the best answer.
In addition, medical device companies are becoming "close to the people", Philips has both 2B business and 2C business, Philips has now opened up the medical business and personal health business, began to integrate into a health technology company.
The path that Medtronic built was based on core products and integrated solutions. This logic is well understood, just as the original function of a mobile phone is to socialize on the phone, but the smart phone can combine the functions of taking photos, communication, entertainment, and life management together, and finally tell the user what to do. Medtronic's "artificial pancreas" is like the best communication device, and he is building a smartphone about diabetes. He is also recruiting horses to belong to its "app".
In an interview with MobiHealthNews in 2015, Medtronic President Omar Ishrak said the company will focus on smaller acquisitions and the development of diabetes. In 2014, Medtronic acquired Corventis, a wearable skin care heart monitoring company.
Corventis's tear-off skin patch, the PiiX sensor, was approved by the FDA in 2009. The PiiX measures a range of vital signs, including fluid status, heart rate, HRV, respiratory rate, activity, and posture.
Hunting of the giants
The arterial network found that collecting user data for effective diabetes management is a long-term arrangement for IT giants such as Google and Apple, and multinational pharmaceutical companies such as Sanofi. Google's approach is through cooperation with professional pharmaceutical companies or devices, while Apple is a secret layout.
At the beginning of the article, Medtronic's competitor Dexcom developed a bandage-sized glucose monitor in 2015 with Google. Many digital health projects are not yet rewarding, but the cooperation between Dexcom and Google shows that the results are good.
On November 20th, Dexcom expressed its willingness to continue to pay the renewal fee. It has signed a revised licensing agreement with Alps' life sciences division, Verily, to accelerate Dexcom's research and development in type 2 diabetes testing. Under the revised agreement, Dexcom needed to provide Verily with a $250 million advance payment and a subsequent payment of $280 million in milestone payments.
Previously, Verily also cooperated with Sanofi to create a joint venture company, Onduo, and Google's Verily and Sanofi jointly invested $500 million. Both parties designed a fully functional diabetes virtual clinic on Onduo.
Apple, in 2017 foreign media reports, Apple has a secret biomedical engineer team responsible for developing sensors to monitor blood sugar levels, and this great plan is Jobs's last wish, if this plan is successful, then Apple watch Equipment will become a necessity for people with diabetes.
According to three people familiar with the matter, they are part of Apple's super secret program to develop sensors for non-invasive continuous blood glucose monitors.
According to CNBC, such a breakthrough will become the "Holy Grail" of life sciences. Many life science companies have tried and failed because it is very challenging to accurately track glucose levels without piercing the skin. It is also reported that Apple has been conducting feasibility tests in clinical facilities in the Bay Area and has hired consultants to help it find regulatory channels.
A person familiar with the matter said that about a year ago, about 30 people were working in the organization. But since the company has dug away from companies such as Vital Connect (wearable vital signs company), Masimo (non-invasive blood oxygen monitoring), Sano (blood glucose monitoring), Medtronic (Cedtronics) and C8 Medisensors (optical blood glucose dynamic monitor) Since about 12 biomedical experts, various speculations have emerged. According to sources, some of them joined the secret blood sugar monitoring team, while others joined the Apple Watch group.
FDA has opened a green light, domestic pharmaceutical companies try to deepen
In 2015, there was also a phenomenon of diabetes management APP in the “Drinking Sugar†in the domestic diabetes field, and there were not many companies that survived. The main business models of the still active Diabetes APP are: medical e-commerce, cooperation with pharmaceutical companies and device manufacturers, exploring foreign HMO models, namely insurance + medical + data, online and offline management of patients, to patients Charge a certain membership fee, pay for cooperation with B-side companies, etc.
Tencent launched its own smart blood glucose meter in 2015: “Teng Ai Sugar Doctorâ€. At the beginning of 2017, Tencent and Lilly and Lilac Park reached a cooperation to launch the “Greece Diabetes Excellence Care Project†for diabetes care and support projects.
The role of domestic diabetes management is relatively active, pharmaceutical companies, the arterial network has counted 18 home chronic disease management to change the channel to the pharmaceutical companies. It is found that more than half of the pharmaceutical companies have cooperated with e-commerce, Internet medical platform, and chronic disease management APP to conduct services in a cooperative manner; Internet platforms, intelligent hardware, and offline services are among the top three in the form of chronic drug service.
In domestic health management is generally a new thing, the entry threshold is low, there is no clear restrictions on regulations. However, in foreign countries, products that want to obtain market approval must pass the FDA. Fortunately, the FDA is still generally green, open-minded and continuously improve the regulations.
About a year ago, the FDA announced its pre-certification pilot program, which will shift the focus of regulation from a specific product to the company and developer of the product. The idea is that if the FDA is convinced that a company is responsible and safe in its development process, then it does not need to regulate all products.
In late June, the FDA issued its second draft of its pre-certification framework, which lists the 12 categories that the agency can refer to when evaluating an organization. These lists include leadership, transparency, people, and risk management.
In April of this year, a 17-page first draft was published. The first draft lists the details of the software pre-certified as a medical device (software as a medical device SaMD) two levels, one for experienced companies have developed SaMD ready and the other is for the first time the development of enterprise-ready SaMD .
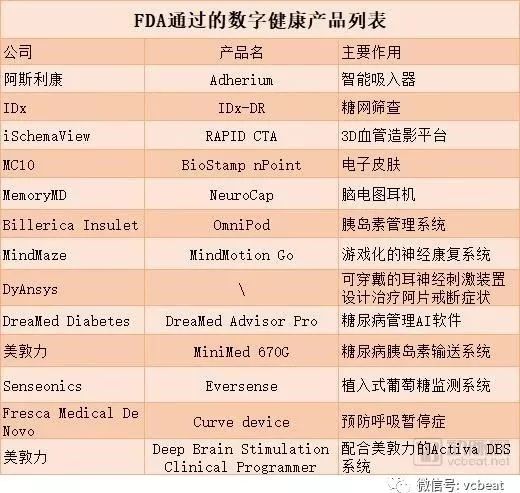
For the quarter, the FDA has adopted 14 products, which shows the FDA's attitude. A list of products approved by the FDA this quarter is attached:
Biological Microscope ,Microscope For Biology Students,Microscope For Biology,Biology Lab Microscope
Ningbo ProWay Optics & Electronics Co., Ltd. , https://www.proway-microtech.com