Through the blood-brain barrier, enzyme replacement therapy can improve cognitive function in patients
Through the blood-brain barrier, enzyme replacement therapy can improve cognitive function in patients
February 11, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];ArmaGen, a biotechnology company that develops treatments for severe neurological diseases, recently revealed a 52-week complete result of a proof-of-concept phase 2 clinical study. AGT-181 is a research therapy for the company's type I mucopolysaccharidosis (MPS I, also known as Hurler-Scheie syndrome). Data released at the 14th annual WORLDSymposium conference in San Diego, USA, showed that AGT-181 stabilized the neurocognitive development quotient (DQ) of patients with severe MPS I. These data validate previous findings and demonstrate that ArmaGen's patented drug delivery technology delivers biopharmaceuticals through the blood-brain barrier (BBB), providing therapeutic benefits for patients with severe MPS I.

MPS I is a rare hereditary lysosomal storage disease caused by the lack of Idusidase (IDUA), which breaks down complex carbohydrates produced by the body. MPS I affects approximately 3,000-4,000 patients worldwide. MPS I affects a variety of organs including the brain, including stunting, mental decline, loss of body function, impaired language development, airway obstruction, corneal and retinal damage, carpal tunnel syndrome, and body movements that limit joint movement And neurological symptoms. Mild MPS I includes Hurler-Scheie and Scheie syndrome, and patients with Hurler-Scheie syndrome may have mild cognitive impairment or attention problems. Patients with Scheie syndrome usually have late-onset, mild symptoms and slower disease progression, usually without involving the central nervous system, but may have significant morbidity.
AGT-181 is a novel research enzyme replacement therapy for the treatment of physical and cognitive symptoms in patients with MPS I. ArmaGen developed AGT-181 to target the insulin receptor by reconstituting the fusion protein of IDUA binding to immunoglobulin G (IgG) antibodies. Using ArmaGen's proprietary "Trojan Horse" technology, AGT-181 utilizes the body's natural system to non-invasively deliver proteins and other macromolecules across the blood-brain barrier (BBB).
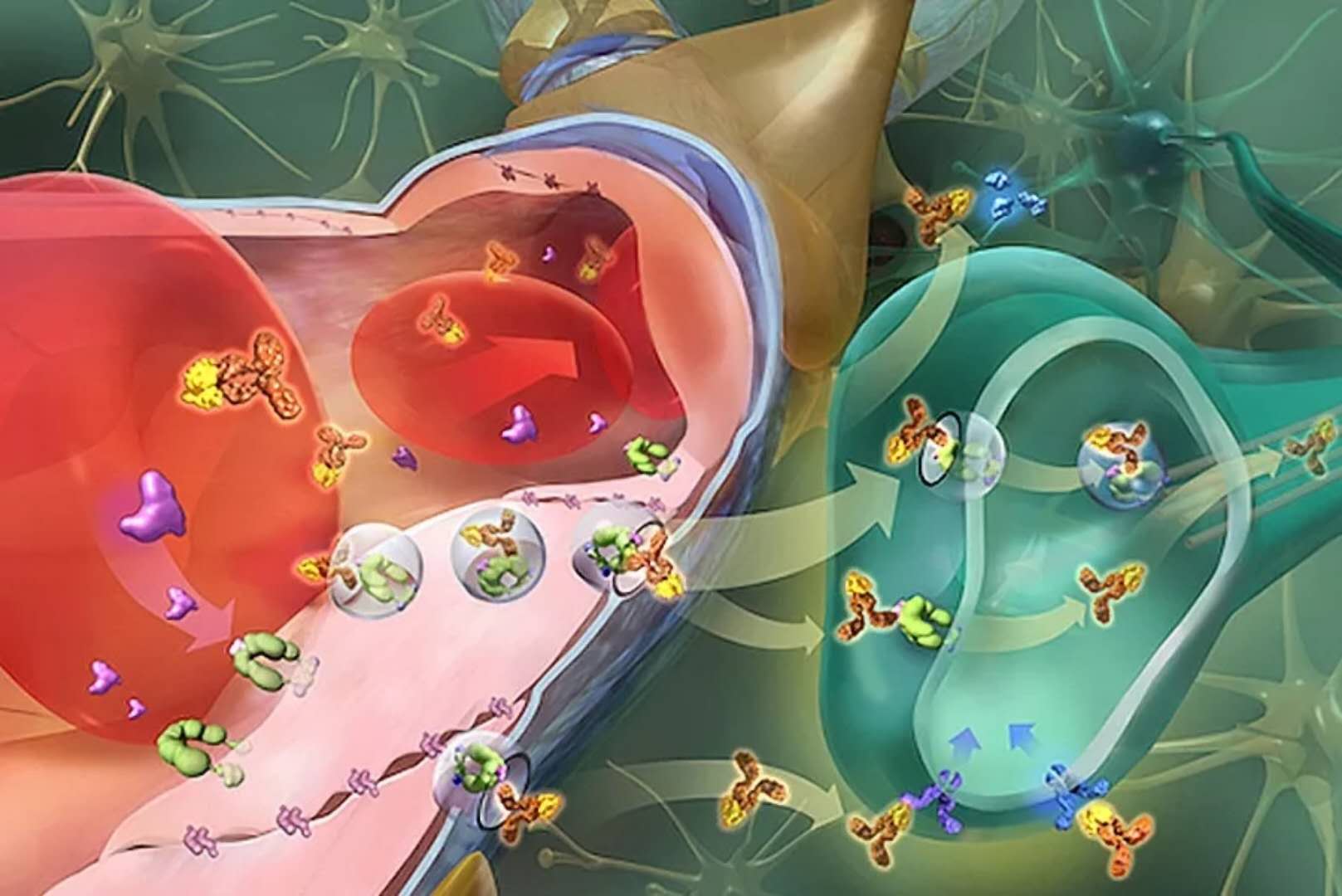
â–²ArmaGen's technology can help drugs penetrate the blood-brain barrier (Source: ArmaGen official website)
At the 13th WORLDSymposium annual meeting in 2017, Dr. Giugliani of the Hospital de ClÃnicas in Porto Alegre Hospital in Brazil presented age-related cognitive functions after a six-month, two-phase clinical study of five MPS I children. Preliminary evidence of improvement. This year's report includes 52 weeks of data for all 11 participants (aged 2 years and older). Nine of the 11 patients underwent previous enzyme replacement therapy and one received a stem cell transplant with failed implantation. In these trials, these children received an intravenous infusion of AGT-181 at a dose of 1.0, 3.0 or 6.0 mg/kg per week. The researchers performed a neurocognitive test that was appropriate for developmental age at 13, 26, and 52 weeks of treatment. The study used the following validated neurocognitive assessment test: Bayley Infant Development Assessment Scale, Third Edition (BSID-III) ) or the Kaufman Children's Complete Assessment Test (KABC-II) and the Vineland Adaptive Behavior Scale Second Edition (VABS-II). Overall, these tests represent a score that reflects cognitive, linguistic and motor skills.
Dr. Giugliani reported a stable cognitive DQ in the patient who did not have a net decrease in DQ after 52 weeks of receiving AGT-181. He also reported a steady volume of cortical gray matter after 52 weeks of treatment with AGT-181. In nine patients who had been treated with lanitase ERT for 1-12 years, AGT-181 may be involved in further improvement in somatic disease control based on further reductions in liver and spleen volume and increased shoulder mobility and range of motion. In addition, the anti-drug antibody (ADA) data for AGT-181 treatment was comparable to the lanitase. AGT-181 was well tolerated and the researchers did not identify serious adverse events that may be associated with the drug.
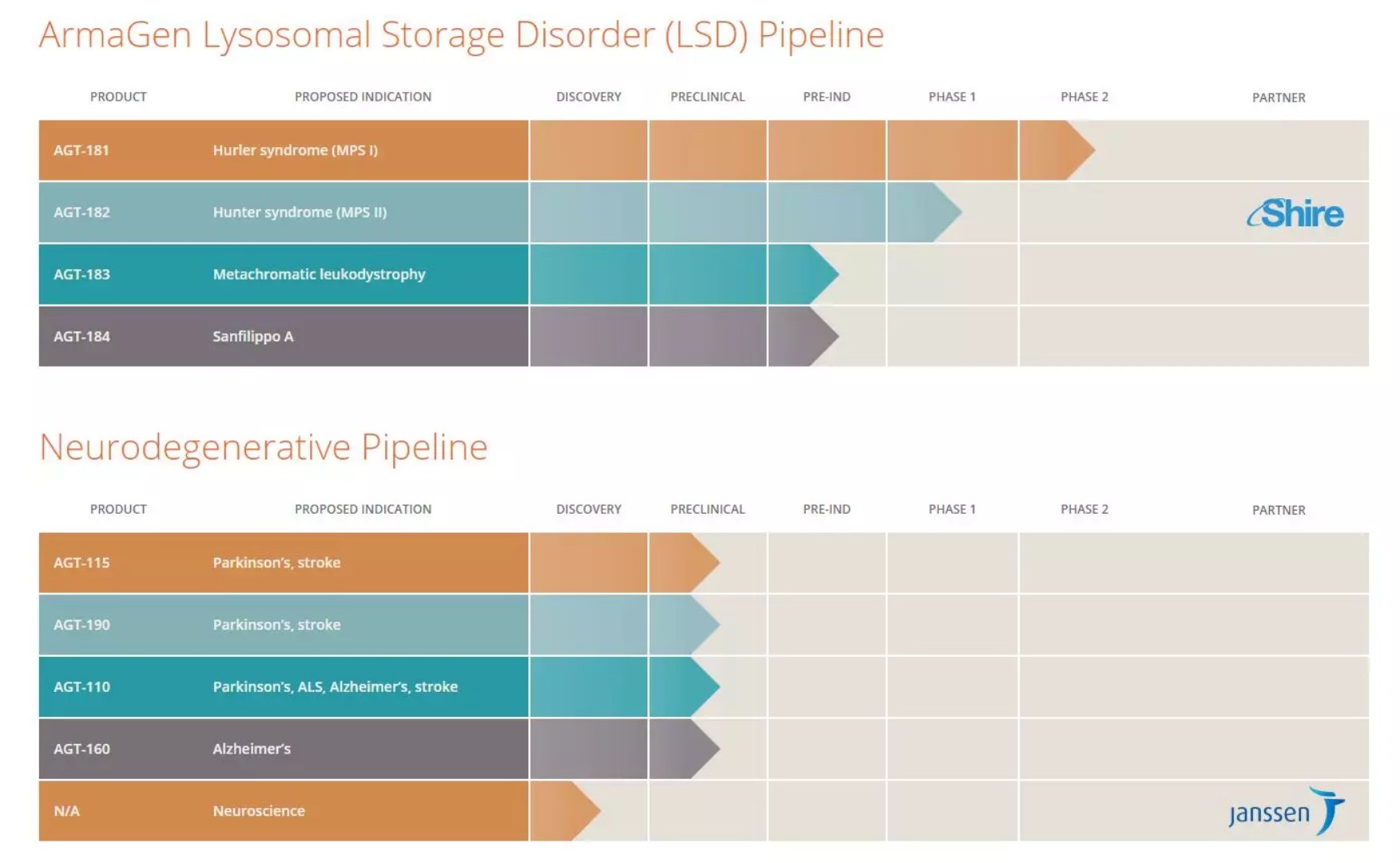
â–²ArmaGen's R&D pipeline (Source: ArmaGen Official Website)
Dr. Giugliani said: "The results of this phase 2 clinical study validated the AGT-181 crossing the blood-brain barrier and benefited the initial results of neurocognitive function in children with severe MPS I. Existing enzyme replacement therapy improved MPS The somatic performance of I patients, however, they cannot cross the blood-brain barrier and thus cannot resolve severe and progressive neurological symptoms. Since we have evidence of conceptual inflammatory disease, a controlled phase 3 clinical trial is needed to test The long-term cognitive impact of drugs on MPS I patients with central nervous system involvement."
"By confirming our initial findings on the stability and improvement of neurocognitive behavior in children with MPS I, the full results of the Phase 2 proof-of-concept trial, together with our recent fast-track identification from the FDA, have enabled us to provide new treatments for MPS I patients. The goal of the selection," Dr. Mathias Schmidt, CEO of ArmaGen, said: "The latest results add more and more evidence that our proprietary "Trojan Horse" technology can deliver missing enzymes to the patient's central nervous system, And it is conducive to neurocognitive function. We are very grateful to the patients and their families involved in the Phase 2 clinical study."
We expect this new drug to enter Phase 3 clinically and bring health to children with this rare and serious disease.
Reference materials:
[1] ArmaGen's AGT-181 Demonstrates Neurocognitive Benefit in Children With Severe MPS I
[2] ArmaGen official website
Low Sugar Fiber,Sugar Reduced Fiber,Energy Bar Fiber,Indigestible Dextrin Fiber
Qingdao Bailong Huichuang Bio-tech Co., Ltd. , https://www.qdblcycn.com